Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
02 ScienceWritingHeuristiChemistry
Caricato da
Fehmeed AlchemyDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
02 ScienceWritingHeuristiChemistry
Caricato da
Fehmeed AlchemyCopyright:
Formati disponibili
In the Laboratory
Implementing the Science Writing Heuristic in the Chemistry Laboratory
K. A. Burke and Thomas J. Greenbowe* Department of Chemistry and Department of Curriculum and Instruction, Iowa State University of Science and Technology, Ames, IA 50011-3111; *tgreenbo@iastate.edu Brian M. Hand Department of Curriculum & Instruction, University of Iowa, Iowa City, IA 52242
Over the past twenty years, educational research studies have shown that if a traditional laboratory report format is used with traditional verification activities, students may learn laboratory techniques, but they learn little else (13). To address this problem, inquiry and collaborative learning have been used as instructional strategies in college introductory chemistry laboratory (47) and organic chemistry (811) courses. In a guided-inquiry format, students are not told explicitly how to do the experiment; they are required to be more active in designing the experiment than with a traditional format. Students who use inquiry procedures are more likely to understand what they have done and why they have done it because they have proposed a hypothesis, designed an experiment to test this hypothesis, collected data, observed trends, and made connections between observations and principles (1214). Guided-inquiry experiences help students think about the ideas underlying laboratory work, construct concepts to answer open ended-questions, and engage in independent thought (1520). Studies of the use of inquiry approaches in college science laboratory courses (21) provide evidence that these strategies
Are more student involved and more inductive than more traditional approaches; Provide less direction and therefore assign students more responsibility to determine procedural strategies; Encourage students to make more use of science process skills; Produce significantly greater educational gains than traditional laboratories; Appear to work equally well for college students of all ability levels, not just the very academically talented; and Appear to be preferred by students.
instructional technique that combines inquiry tasks, collaborative work, and writing, while providing a structure for both students and instructors to do effective activities in the chemistry laboratory. The SWH provides a structure for creating a classroom dynamic between the instructor and the students that promotes inquiry learning and leads students to understand the concepts under investigation. Justification for the Science Writing Heuristic Experiencing and understanding scientific phenomena and the scientific process are goals of most science laboratory courses. To achieve these goals laboratory courses should provide an opportunity for students to restructure information rather than simply be involved in verifying what they have been told. Students need to actively construct science knowledge (27, 28) by being purposefully involved in posing questions, determining claims, and providing evidence. Framed around the use of inquiry tasks, activities that are designed to engage students to think about the ideas underlying laboratory work have proven to be effective (19, 29). Collaborative work and writing are two techniques that have been used to engage students to think explicitly about the ideas underlying their laboratory experiment (30, 31). Collaborative learning has been shown to benefit students sociologically, personally, and academically (32). Research has also shown that students are more engaged with their learning goals when placed in an interactive task-oriented situation than during a lecture or traditional laboratory format (33). Cooper suggested that students involved in effective group collaboration work to construct or comprehend challenging concepts together (34). In a nonthreatening environment, they are able to negotiate their understanding by verbalizing their reasoning, that is, by explaining how they know what they know. Writing is an important component of the language and learning of science (3538): without language there is no science. However, as Langer and Applebee (39) reported, science instructors (as opposed to instructors in other subjects) have their students use writing activities focusing on factual rather than conceptual information. When a writing task is structured to promote the development of conceptual understanding rather than recalling facts, knowledge transformation can occur (40); that is, the concepts about which the student is writing are transformed into a new and more enriched version than before the writing. The act of writing becomes one of learning rather than knowledge-telling where the writer purposefully links new ideas to prior knowledge to build understanding of the concepts being studied.
However, just because students are using an inquiry laboratory manual (cf., 22, 23 ) does not guarantee that students are doing inquiry or that the instructor is teaching using the inquiry process (24, 25). Herron and Nurrenbern (26) stated that, Inquiry-oriented laboratory activities teach inquiry better than lecture demonstration or verification lab exercises, but only if teachers are skilled in inquiry teaching methods and students are given the time and guidance required to become comfortable with the new methods and expectations (p 1358). Our goal is to provide information about how to implement the Science Writing Heuristic (SWH), an
1032
Journal of Chemical Education
Vol. 83 No. 7 July 2006
www.JCE.DivCHED.org
In the Laboratory
Table 1. Comparing Nonproductive and Productive Beginning Questions Nonproductive Beginning Questions Why is the experiment performed in an evaporating dish, not a crucible? Why is the reactant red? Why is the product black? What is the mass of the product? Productive Beginning Questions Is there a relationship between the initial mass of reactant and the final formula of the product? Does the mass of oxygen reacting depend on the original mass of reactant? Does the empirical formula of the reactant depend on the mass of reactant?
copper, they heat the reactant to form black copper(II) oxide. The students are cautioned to carry out the experiment in a ventilated hood space to avoid inhalation of any starting material. They are further alerted to the dangers of hot porcelain or hot iron lab ware. Specific detailed instructions for doing an experiment are not provided to students. Students, then, gather to exchange thoughts about the laboratory exercise. During this time, they
Discuss beginning questions (as a class); Assign their own work groups (usually pairs or groups of four, depending on the task); Decide what data to gather; Prepare a classroom data grid to be completed by the different collaborating groups; and Determine among themselves which group(s) should be responsible for individual taskscollecting original data, making replications, and so forth.
A critical component of the writing process as outlined by Rivard and Straw (41) is the importance of student sharing, clarifying, and distributing knowledge among peers. Asking questions, hypothesizing, explaining, and formulating ideas during peer discussions are necessary precursors for writing. Furthermore, they found that when the techniques of student discussion and individual student writing are combined, students achieve a deeper realization of the target concept(s) and that these concepts are retained over time. An Overview of the Science Writing Heuristic The Science Writing Heuristic, SWH, is both an alternative format students use for their laboratory reports, and a teaching model used by the instructor to help guide the flow of activities associated with an experiment (17, 24, 4245). A heuristic can be a guide or a method used to help individuals or people to discover or reveal something. The SWH engages students in collaborative inquiry activities, negotiation of conceptual understanding, and individual writing and reflection (39, 4648). The SWH template encourages students to talk about, deliberate, and negotiate their understandings of chemistry. The SWH also provides a template for instructors to help students think through, and build understanding of the subject matter, rather than merely following a recipe (49, 50). Using the SWH, instructors actively guide students to help them understand what they are doing, why they are doing it, and to develop conceptual understanding. The SWH strategy mirrors the processes of dialogue and argumentation that scientists use to construct a theory or a concept. The SWH technique is consistent with the pedagogical and philosophical view of doing inquiry learning as outlined by Spencer (14). Components of the Science Writing Heuristic
Beginning Questions
At the outset of the term, students participate in training exercises as an entire group to help them formulate beginning questions (51). These questions are researchable and must be able to be answered by doing an experiment (Table 1). Prior to arrival at the laboratory, students read preview material about the collaborative inquiry activity where a problem is posed to the students. Students are not overtly told what to do or how to do it. For example, the laboratory activity mentioned in the previous section starts with the following scenario: The label has come off the bottle of a red powder in the laboratory. Heating the red powder results in a product that is black copper(II) oxide. Is the red powder pure copper or an oxide of copper? From this scenario, students think about what aspects of this activity they would like to explore and draft one or more beginning questions that will frame their investigation. An SWH laboratory manual provides information about what materials and equipment are available to do an investigation (5254). At the start of the laboratory session, students write their questions on the chalkboard. The group discusses which one(s) should become the class project. This freedom of choice promotes greater student engagement and motivation (24). Once they have decided on a strategy, students divide themselves into work groups to investigate aspects of the problem(s) posed by the beginning question(s). By having the students write their own beginning questions and then having them design their own experimental procedure to answer their question(s), they are more likely to be able to explain to the instructor or to write about what they are doing and why they are doing it. Students value beginning questions because formulating them helps students to focus on the important aspect(s) of the lab exercise as well as organize themselves to perform the lab work.
Prelaboratory Discussion A prelaboratory discussion among the students and the instructor highlights special safety or procedural concerns. Students need to understand the safety requirements and any laboratory techniques; therefore, safety instructions and laboratory techniques are explicitly taught. For example, during an experiment in which students determine whether a red powdery starting material is pure copper or some oxide of
Tests and Observations
Student groups work together to draft appropriate data compilation tables on the chalkboard, then begin collecting experimental information. Students typically work in groups
www.JCE.DivCHED.org
Vol. 83 No. 7 July 2006
Journal of Chemical Education
1033
In the Laboratory
of four. The instructor moves among groups, keeping the students on task, asking guiding questions, or redirecting student inquiries to classmates. As each group generates data, the information is recorded in the class data table. This is important because each group will not likely be reproducing exactly the same procedure as their neighbors. Groups can decide to divide up the tasks, vary the ratio or quantities of materials used, or both. This requires that all students examine patterns or trends arising from the overall experiment. They may do this in their work groups or in conjunction with the entire class. At the conclusion of a collaborative laboratory activity, students engage in a classwide discussion about what the outcome of the experiment is. Because the students have decided together which beginning question(s) to investigate and how to conduct their studies, they are comfortable talking with one another about results they have obtained. They are also eager to offer their suggestions as to why there may be anomalies in the data. The SWH is designed to promote classroom discussion during which individual student explanations and observations are compared and confirmed or contested against the insights and support of the entire group. This is accomplished when students construct their ideas via peer discussion, argumentation, and negotiation. The instructor facilitates but does not lead the discussion.
Supporting a claim with evidence assists students in sorting out the data to answer the beginning question(s). One student observed, It helps to explain the raw data and put it into context. Another noted, It helps put calculations into words. Finally another student said, It helps you to understand when you have to put it on paperit makes you think how to explain. Those students who work more quickly than others are encouraged to make claims and support them with evidence while waiting for slower groups to finish their experimental work. Ideally, all groups would make and discuss claims prior to leaving the laboratory. Ultimately, this is a function of the time remaining at the end of the laboratory session after the class discussion.
Reflections
The reflections section in the SWH format encourages students to think about how their own ideas have changed by looking back on the laboratory activity. A part of this may be accomplished in the lab room with group mates or during the postlab class discussion. Students can then complete the reflection section for the report outside of class. By completing this section on their own, students are more likely to process and understand what they have done in the laboratory. Students ideas can change through the course of doing their experimental work as the students understand more about the concept they are exploring or their ideas might change at some later point in time, after thinking more about their experiences. This can be seen in the following student comments:
Sometimes I was right on with what I was thinking, sometimes I did a 180. Sometimes my results did not match my predictionsI had to explain it to myself. A lot of times I started out with one goal and ended up solving more than just my first goal. When a prediction was proven wrong, I remembered that I was wrong and why.
Claims and Evidence
Students are encouraged to make explicit associations among beginning questions, observations, data, claims, and evidence as well as to be able to defend their position. When students make a claim for an investigation, they are expected to note a pattern, demonstrate a generalization, articulate a relationship, or provide an explanation that they have uncovered by their work. Such activities encourage a greater sense of ownership among the studentsthey are not merely following a step-by-step model to complete their weekly cookbook procedure and corresponding report. By being able to state a claim and provide rational evidence for their claim, students display scientific argumentation and reasoning skills. The knowledge being discussed is their knowledgethey are required to test their knowledge and understanding against what the expectations of the activity are. By doing an inquiry laboratory activity, students have the opportunity to reflect on the concepts that are embedded in that activity and are more likely to learn these concepts (55) than if they had simply verified a concept. Continuing to explore the example cited earlierconverting a red copper powder to black copper(II) oxidea student claimed, The empirical formula for the unknown red compound does not depend on the mass of that compound used in the experiment. The experimenter cited supporting evidence: Class members experimented with masses of the starting compound in the range of 1.0000 to 3.0000 grams. After they reacted the compound with oxygen in the air, they determined the empirical formula for the starting compound to be the same, Cu2O, no matter what mass of the starting compound they used. Students view the writing of claims and evidence as an opportunity to re-examine work in lab and what was learned.
1034 Journal of Chemical Education
When students can explain what they think they know and justify their understanding, it becomes obvious that they have learned something and are not merely providing a rote reply. For example, in the copper oxide experiment, many students predict that the reactant will lose mass when heated. Some do not think the statement that The empirical formula for the compound is the same no matter what the starting masses of the reactants are... could possibly be supported by the data. Upon further reflection, students realize that their experiment does support the statement and is a good example of the law of constant composition. Students believe that their reflections play a large part in their understanding. They find that reflections make it easier for them to realize whether they grasp what they do in the laboratory. Further, writing reflections helps them to make a connection to the lecture and understand the concepts behind their lab work. As one student stated, It helps me on exams in lecture. Finally, Reflections helped me think about what to do differently if I would do the experiment again.
www.JCE.DivCHED.org
Vol. 83 No. 7 July 2006
In the Laboratory
The Science Writing Heuristic Laboratory Report The SWH laboratory report format promotes students participation in laboratory work by requiring them to frame beginning questions, propose methods to address these questions, and carry out appropriate investigations. It also prompts them to share and compare their laboratory findings with their peers and with information in a textbook, on the Internet, or from other sources. The SWH laboratory report provides a format to guide student discussions, thinking, and writing via beginning questions, claims and evidence, and reflections (Table 2). A carbonless laboratory notebook with alternating white and yellow quadrille pages is used for the SWH reports (56). Although making observations in the SWH format may be similar to traditional verification work, the SWH laboratory report format requires students to make claims (draw inferences) and support the claims with evidence from experimental work. The SWH helps students think about the relationships among questions, evidence, and claims, which is much more demanding cognitive work than simply verifying that the results obtained from the experiment match the accepted value reported in the literature. The reflections part of the SWH laboratory report encourages students to think how the science activity relates to their own prior knowledge and how their understanding of that topic has changed. An SWH laboratory report and class differ from a traditional laboratory report and class. The SWH report is written with the goal of answering a series of questions designed to help the students understand the experiment they are doing and the underlying concepts associated with the experiment. The whole class votes on which beginning questions will be explored, which experiments will be done, and how they will be done. Students negotiate what they will do and why they will do it. Through discussions with their peers about which beginning questions are worth pursuing, which
Table 2. Comparing Student Report Formats for the Science Writing Heuristic (SWH) and the Traditional Laboratory Traditional Report Format Title, purpose. Outline of procedure. Data and observations. Discussion. Balanced equations, calculations, and graphs. SWH Student Template Beginning QuestionsWhat are my questions? TestsWhat do I do? ObservationsWhat can I see? ClaimsWhat can I claim? EvidenceHow do I know? Why am I making these claims? ReflectionsHow do my ideas compare with other ideas? How have my ideas changed?
optimum experimental design should be used to answer the questions, and which evidence supports the claims made on the basis of the data collected, the purpose of the time spent in the laboratory changes dramatically for both students and the instructor. The whole class is involved in sharing data and analyzing the data. Each student is responsible for his or her SWH laboratory report; however, the data and their interpretation are decided by interactions with other students and the instructor. Everyone in the class is responsible for helping their classmates gain an understanding of the concept(s) being explored by the laboratory exercise. Because the SWH laboratory report format is new to both students and instructors, we developed a grading rubric to help students write their reports and to help instructors grade the reports. Individual laboratory reports are graded using a seven-category, 30-point grading rubric. An abbreviated version of the rubric is shown in Table 3. Students are provided a thorough verbal explanation as a part of the first
Table 3. SWH Grading Rubric for Instructors Section of Report Beginning Question(s) Safety Considerations Categories What question(s) did I have? What question(s) did the class group decide to use? What general point(s) can I make about staying safe in this experiment? What more specific point(s) should I make about a certain chemical or procedure? What did I actually do (in outline form, specific enough for someone else to follow) to perform this experiment? Data, Observations, Calculations, and Graphs What qualitative observations did I make? What quantitative data have I collected, and what calculations did I do to make sense of my data? What balanced equations have I written? Have I prepared a properly labeled and titled graph? What can I claim to answer my beginning question(s) or the classs beginning question(s)? Evidence and Analysis Reading, Reflection, and Post-Laboratory Questions What is my interpretation of my data (graphs, class data, trends, or other analysis to support my claim(s)? Have I connected the proper evidence with the proper claim? (a) Have I identified and explained sources of error and assumptions made during the experiment? (b) How have my ideas changed, what new questions do I have, or what new things do I have to think about? (c) How does this work tie into concepts about which I have learned in class? (d) To what can I refer in my text, my notes, or some real life application to make a connection with this laboratory work? (e) What are my answers to any postlab questions? How do I incorporate them into my report? Number of points 02 2 2 06
02 6 10
NOTE: Total of 30 points for each laboratory.
www.JCE.DivCHED.org
Vol. 83 No. 7 July 2006
Journal of Chemical Education
1035
In the Laboratory
laboratory session and an extensive written explanation in their laboratory manual (54) of how the points are awarded so that they are well-informed about how detailed their reports should be. The instructor completes an individual rubric grid for each students report. By referring to the grid and the annotated explanation of the grading rubric, the student is aware of how well the submitted work fulfills the criteria of an acceptable SWH report and where improvements might be made. The Role of the Instructor The instructors goal is to facilitate students progress without dictating procedures and approaches or directly answering questions (55). Instructors encourage students to use interactive constructivist techniques (where meaning is interactively socially constructed as well as personally constructed) (57, 58) to frame their questions, hypotheses, and experimental designs. Instructors guide or coach, but do not lead. The more students are able to make decisions, the more ownership, responsibility, and accountability they feel towards the laboratory activity. They become more engaged, are more motivated, exert more effort, are more interested in the outcome, and consequently learn more as a result (24). Instructors need to assist students in negotiating meaning from experimental data and observations. Collaborative intra- and intergroup discussions provide ample opportunity for socially constructing concepts or ideas by making claims (drawing inferences) and supporting them with evidence from their experimental work. The socially constructed view of claims is focused around patterns of analysis; that is, students are not just generating and constructing from their own ideas but from the ideas of entire class. Effective instructor strategies for implementation of the SWH are shown in Table 4. The instructor creates a student-centered learning environment and encourages students to assume primary responsibility for their own learning.
Assessment and Evaluation of the Science Writing Heuristic Writing is an important component of the learning process and an important component of assessing students understanding of chemistry. The SWH strategies include writing exercises that expand a students opportunity to engage with the ideas of science, rather than perceiving science writing as note-taking, fill-in-the-blank, or factual regurgitation kinds of exercises (45). Writing-to-learn tasks require students to deal with science knowledge and the practice of science and to begin to recognize what it means to construct science knowledge and the reasoning strategies necessary to accomplish this (5961). Students using writing-to-learn experiences gain an understanding of science and the inquiry process better than students with traditional writing experiences (48). The more students are encouraged to think, the more they take responsibility for their own learning; therefore, opportunities for writing experiences promotes greater equity among the students (48). Hohenshell found that the SWH approach guides all students to think and reason at a higher level, and this closes the gap between the high achieving and low achieving chemistry students. Students who participate in the SWH approach are better able to design an experiment to address a hypothesis compared to students who participate in a traditional cookbook laboratory activity, as measured on laboratory practical examinations tasks (24). They are better able to evaluate the guided-inquiry process they performed, as well as propose changes to the existing procedure (62). The SWH has been effectively incorporated into various curricula (including biology, chemistry, general science, geology, physical science, and physics) from elementary through post-secondary levels (at two- and four-year institutions) (6, 12, 18, 42, 4648, 51, 63). The two lead authors for this article have used the SWH for six semesters in a college general chemistry laboratory course for science and engineering students and for two semesters in a college general
Table 4. Comparison of Less and More Helpful Instructor Laboratory Strategies Area Beginning questions (BQs) Less Helpful Approach There are no BQs or the TA drafts one or more BQs for the students. There is little or no discussion about BQs. TA or laboratory manual outlines exactly what mass, volume, step-by-step process, etc. to use. TA demonstrates each calculation. More Helpful Approach Each pair of students discusses BQs from prelab. Each pair writes one BQ on the chalkboard. There is a class discussion and the BQs are combined or modified. The entire class decides which BQs and parameters to investigate. TA suggests a range of values and students choose their own values from the range. Students create a chart to collect class data. TA suggests that the students talk in small groups about what they have found and asks the students what information they are trying to determine and how they will go about finding it. TA tries to get all students to a point of understanding in order to discuss their results. Student help their peers to understand how to complete their work and develop claims and support them with evidence. Students ponder the data, analyze appropriate calculations, and talk with one another to try to make meaning of the data that have been collected and what implications the data have for their studies. They discuss trends and anomalies in data, construct concepts, answer beginning questions, make claims, and outline supporting evidence.
Laboratory strategies
Calculations
Class discussion
TA summarizes the trends and results students have found and explains the point of the laboratory exercise.
1036
Journal of Chemical Education
Vol. 83 No. 7 July 2006
www.JCE.DivCHED.org
In the Laboratory
chemistry laboratory course for agriculture, biology, consumer family science, and exercise physiology majors. The SWH decreases the quantity of time students spend writing their reports and the quantity of time instructors spend grading reports (63). In another study, the SWH approach was found to foster a classroom dynamic that improved student performance on chemistry lecture exams and on conceptual understanding compared to students using an inquiry laboratory manual but not doing the SWH effectively (25). Students perceive that their enhanced conceptual understanding in the laboratory leads to improved performance on lecture quizzes and examinations. Replying to an end-ofsemester survey about their laboratory experiences, students cited at least four laboratory experiences that directly helped them correctly answer questions in a testing environment. Importantly, effective implementation of the SWH by both students and their instructors enabled the difference in the achievement gap between males and females to be closed. Females averaged lower scores on an ACS diagnostic test compared to males. At the end of the course, females average scores were similar to males on total points earned in the course and on an ACS examination (24, 25). Summary The Science Writing Heuristic is a structured learning tool that requires both students and instructors to be active and interactive in the laboratory experience. It is a process that blends inquiry and writing practices to assist students to better understand chemistry through laboratory activities. Rather than view laboratory activities as cookbook verification work, students are expected to engage in the processes of science; that is, they need to pose questions, collect and analyze data, make claims based on thought-out evidence, and defend their answers among their peers. The more that instructors can encourage this inquiry-based approach, the more involved and sophisticated the students writing in their laboratory reports will become. This will in turn lead to richer understanding of the chemistry concepts under study. Literature Cited
1. Hofstein, A.; Lunetta, V. N. Rev. Educ. Res. 1982, 52, 201 217. 2. Lazarowitz, R.; Tamir. P. Research on Using Laboratory Instruction in Science. In Handbook of Research on Science Teaching and Learning; Gabel, D. L., Ed.; MacMillan: New York, 1994. 3. Nakhleh, M. B.; Polles, J.; Malina, E. Learning Chemistry in a Laboratory Environment. In Chemical Education: Towards Research-Based Practice; Gilbert, J. K., DeJong, O., Justi, R., Treagust, D. F., Van Driel, J. H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp 6994. 4. Todd, D.; Pickering, M. J. J. Chem. Educ. 1988, 65, 1100 1102. 5. Burns, D. S.; Berka, L. H.; Kildahi, N. J. J. Chem. Educ. 1993, 70, A100. 6. Keys, C. W.; Hand, B.; Prain, V.; Collins. S. J. Res. Sci. Teach. 1999, 36, 10651084. 7. Farrell, J. J.; Moog, R. S.; Spencer, J. N. J. Chem. Educ. 1999, 76, 570574.
8. McElveen, S. R.; Gavardinas, K.; Stamberger, J. A.; Mohan, R. S. J. Chem. Educ. 1999, 76, 535536. 9. Centko, R. S.; Mohan, R. S. J. Chem. Educ. 2001, 78, 7779. 10. Horowitz, G. J. Chem. Educ. 2003, 80, 10391041. 11. Mullins, R. J.; Vedernikov, A.; Viswanathan, R. J. Chem. Educ. 2004, 81, 13571361. 12. Mason, D. J. Chem. Educ. 2004, 81, 1241. 13. Olney, D. J. Chem. Educ. 1997, 74, 13431345. 14. Spencer, J. N. J. Chem. Educ. 1999, 76, 566569. 15. Zare, R. N. Chem. Eng. News 2005, 83 (5), 3. 16. Deming, D. S.; Cracolice, M. S. Sci. Teach. 2001, 68, 2024. 17. Hand, B. M.; Prain, V.; Lawrence, C.; Yore, L. Int. J. Sci. Educ. 1999, 21, 10211035. 18. Gunstone, R. F.; Champagne, A. B. Promoting Conceptual Change in the Laboratory. In The Student Laboratory and the Science Curriculum; Hegarty-Hazel, E., Ed.; Rutledge: London, 1990; pp 159182. 19. Oliver-Hoyo, M.; Allen, D.; Anderson, M. J. Coll. Sci. Teach. 2004, 33, 2024. 20. Keefer, R. J. Coll. Sci. Teach. 1999, 29, 159165. 21. Leonard, W. H. The Laboratory Classroom. In Handbook of College Teaching; Prichard, K. W., Mclaran-Sawyer, R., Eds.; Greenwood Press: Westport, CT, 1994. 22. Abraham, M. R.; Pavelich, M. J. Inquiries into Chemistry; Waveland Press, Inc.: Prospect Heights, IL, 1979. 23. Van Koppen, P. Discovery and Analysis in the Laboratory; Hayden-McNeil Publishing, Inc.: Plymouth, MI, 2002. 24. Greenbowe, T. J.; Hand, B. M. Introduction to the Science Writing Heuristic. In Chemists Guide To Effective Teaching; Pienta, N. P., Cooper, M. M., Greenbowe, T. J., Eds.; PrenticeHall: Upper Saddle River, NJ, 2005. 25. Poock, J. R.; Burke, K. A.; Greenbowe, T. J.; Hand, B. M. J. Chem. Educ., submitted for publication, 2005. 26. Herron, J. D.; Nurrenbern, S. C. J. Chem. Educ. 1999, 76, 13531361. 27. Bodner, G. M. J. Chem. Educ. 1986, 63, 873878. 28. Shiland, T. W. J. Chem. Educ. 1999, 76, 107109. 29. Galbraith, D. Writing as a Knowledge-Constituting Process. In Knowing What to Write: Conceptual Processes in Text Production; Torrance, M., Galbraith, D., Eds.; Amsterdam University Press: Amsterdam, 1999; pp 139160. 30. Smith, M. E.; Hinckley, C. C.; Volk, G. L. J. Chem. Educ. 1991, 68, 413414. 31. Wallace, C. S. Evidence from the Literature for Writing as a Mode of Science Learning. In Writing and Learning in the Science Classroom; Wallace, C. S., Hand, B., Prain, V., Eds.; Kluwer Academic Publishers: Boston, 2004. 32. Nurrenbern, S. C.; Robinson, W. R. J. Chem. Educ. 1997, 74, 623624. 33. Inquiry and the National Science Education Standards: A Guide for Teaching and Learning; Olson, S., Loucks-Horsley, S., Eds.; National Research Council: Washington, DC, 2000. Video and audio examples of implementing the SWH approach can be found at http://avogadro.chem.iastate.edu/SWH/homepage.htm (accessed Apr 2006). 34. Cooper, M. M. An Introduction to Small-Group Learning. In Chemists Guide to Effective Teaching; Pienta, N. P., Cooper, M. M., Greenbowe, T. J., Eds.; Prentice-Hall: Upper Saddle River, NJ, 2005. 35. Britton, J. Language and Learning; Penguin Books: New York, 1970.
www.JCE.DivCHED.org
Vol. 83 No. 7 July 2006
Journal of Chemical Education
1037
In the Laboratory 36. Gee, J. P. Social Linguistics and Literacies: Ideology in Discourses; Taylor and Francis: Bristol, PA, 1990. 37. Halliday, M. A. K. Ling. Ed. 1993, 5, 93116. 38. Halliday, M. A. K.; Martin, J. R. Writing Science: Literacy and Discursive Power; Falmer Press: London, 1993. 39. Langer, J.; Applebee, A. How Writing Shapes Thinking: A Study of Teaching and Learning; NCTE Research Rep. No. 22; National Council of Teachers of English: Urbana, IL, 1987. 40. Bereiter, C.; Scardamalia, M. The Psychology of Written Composition; Lawrence Erlbaum Associates: Hillsdale, NJ, 1987. 41. Rivard, L. P.; Straw, S. B. Sci. Educ. 2000, 84, 566593. 42. Rudd, J.; Greenbowe, T.; Hand, B. J. Coll. Sci. Teach. 2001, 31, 230234. 43. Writing and Learning in the Science Classroom; Wallace, C. S., Hand, B., Prain, V., Eds.; Kluwer Academic Publishers: Boston, 2004. 44. Keys, C. W. Sci. Educ. 1999, 83, 115130. 45. Keys, C. W.; Hand, B. M.; Prain, V.; Collins, S. J. Res. Sci. Teach. 1999, 36, 10651084. 46. Hand, B.; Keys. C. W. Sci. Teach. 1999, 66, 2729. 47. Hand, B. M.; Prain, V. Sci. Educ. 2002, 86, 737755. 48. Hohenshell, L. M. Enhancing Science Literacy through Implementation of Writing-to-Learn Strategies: Exploratory Studies in High School Biology. Ph.D. Thesis, Iowa State University, Ames, IA, 2004. 49. Peters, D. J. Chem. Educ. 2002, 79, 783786. 50. Michael, J. A.; Modell, H. I. Active Learning in Secondary and College Science Classrooms: A Working Model for Helping the Learner to Learn; Lawrence Erlbaum Associates: Mahwah, NJ, 2003. 51. Burke, K. A.; Hand, B. M.; Poock, J. R.; Greenbowe, T. J. J. Coll. Sci. Teach. 2005, 35, 3641. 52. Heideman, S. A.; Greenbowe, T. J. Laboratory Experiments for Chemistry 177L; Iowa State University Press: Ames, IA, 2002. 53. Heideman, S. A.; Greenbowe, T. J. Laboratory Experiments for Chemistry 178L; Iowa State University Press: Ames, IA, 2003. 54. Greenbowe, T. J.; Burke, K. A. Laboratory Experiments for Chemistry 163L; Hayden-McNeil Publishing, Inc.: Plymouth, MI, 2006. 55. Bunce, D. Inquiry Learning: What It Is and How We Do It. In Chemistry in the National Science Education Standards: A Reader and Resource Manual for High School Teachers; National Academy Press: Washington, DC, 1997. 56. Student Lab Notebook, 50 Carbonless Duplicate Sets, Top Bound, ISBN 1-930882-50-5, Hayden-McNeil Publishing, Inc.: Plymouth, MI, 2005. 57. Domin, D. S. J. Chem. Educ. 1999, 76, 543547. 58. Henriques, L. Constructivist Teaching and Learning. Abstracted from A Study to Define and Verify a Model of Interactive-Constructive Elementary School Science Teaching. Ph.D. Thesis, University of Iowa, Iowa City, IA, 1997. 59. Prain, V.; Hand, B. M. Teach. Teacher Educ. 1996, 12, 609 626. 60. Hand, B. M.; Prain, V.; Lawrence, C.; Yore, L. Intl. J. Sci. Educ. 1999, 10, 10211036. 61. Hand, B. M. Cognitive, Constructivist Mechanisms for Learning Science Through Writing. In Writing and Learning in the Science Classroom; Wallace, C., Hand, B., Prain, V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. 62. Berg, C.; Bergedahl, V.; Lundberg, B.; Tibell, L. Intl. J. Sci. Educ. 2003, 25, 351372. 63. Rudd, J.; Greenbowe, T.; Hand, B.; Legg, M. J. Chem. Educ. 2001, 78, 16801686.
1038
Journal of Chemical Education
Vol. 83 No. 7 July 2006
www.JCE.DivCHED.org
Potrebbero piacerti anche
- Edexcel As Chemistry Practice Unit Test1Documento17 pagineEdexcel As Chemistry Practice Unit Test1Mohammed Hussain100% (2)
- Propagating Trees and Fruit Trees: Sonny V. Matias TLE - EA - TeacherDocumento20 paginePropagating Trees and Fruit Trees: Sonny V. Matias TLE - EA - TeacherSonny MatiasNessuna valutazione finora
- Cambridge C1 Advanced Exam Overview PDFDocumento1 paginaCambridge C1 Advanced Exam Overview PDFrita44Nessuna valutazione finora
- Varying ViewsDocumento5 pagineVarying Viewsforevertay2000Nessuna valutazione finora
- Chapter 1Documento24 pagineChapter 1Carlisa Arsolon LangleyNessuna valutazione finora
- Roth 1994Documento27 pagineRoth 1994Muchsin SimpsonsNessuna valutazione finora
- Mind The Fact: Teaching Science Without Practical As Body Without SoulDocumento9 pagineMind The Fact: Teaching Science Without Practical As Body Without Soulsmartzia87Nessuna valutazione finora
- Dissertation On Teaching AidsDocumento7 pagineDissertation On Teaching AidsOnlinePaperWriterSingapore100% (1)
- The Effect of Inquiry-Based Learning Method On Students' Academic Achievement in Science Course PDFDocumento5 pagineThe Effect of Inquiry-Based Learning Method On Students' Academic Achievement in Science Course PDFbjssurya@gmailNessuna valutazione finora
- Physics Lab Course Using Problem-Based LearningDocumento5 paginePhysics Lab Course Using Problem-Based LearningLylaaCliquers SwithD'angelNessuna valutazione finora
- Research: Four Styles of Teaching Science LabsDocumento5 pagineResearch: Four Styles of Teaching Science LabsAnang BudiantoNessuna valutazione finora
- Design-Based Research in Science EducationDocumento15 pagineDesign-Based Research in Science Educationis03lcmNessuna valutazione finora
- Absi English Taufik 2SDocumento6 pagineAbsi English Taufik 2SNurul Rahmiah FitriNessuna valutazione finora
- Inquiry & 5E Instructional ModelDocumento5 pagineInquiry & 5E Instructional ModelMarini Isa100% (1)
- 1 s2.0 S1877042810003678 Main PDFDocumento5 pagine1 s2.0 S1877042810003678 Main PDFChairunnisaNessuna valutazione finora
- Scaffolding Students' Reflective Dialogues in The Chemistry Lab: Challenging The CookbookDocumento11 pagineScaffolding Students' Reflective Dialogues in The Chemistry Lab: Challenging The CookbookMusic HitzNessuna valutazione finora
- Effects of Inquiry Demonstration and Laboratory Work on Students' UnderstandingDocumento20 pagineEffects of Inquiry Demonstration and Laboratory Work on Students' UnderstandingJinky Marie TuliaoNessuna valutazione finora
- A Way To Promote Learning During Laboratory ActivitiesDocumento6 pagineA Way To Promote Learning During Laboratory Activitiesapi-17116217Nessuna valutazione finora
- 10 1002@tea 21082Documento36 pagine10 1002@tea 21082Lia Laily Mukaromatil AhyarNessuna valutazione finora
- The Effect of Implementation of Science Writing Heuristic On Students' Achievement and Attitudes Toward Laboratory in Introductory Physics LaboratoryDocumento5 pagineThe Effect of Implementation of Science Writing Heuristic On Students' Achievement and Attitudes Toward Laboratory in Introductory Physics Laboratoryara miekoNessuna valutazione finora
- 5 Research ArticlesDocumento5 pagine5 Research ArticlesJ. OraaNessuna valutazione finora
- Inquiry & 5E Instructional ModelDocumento5 pagineInquiry & 5E Instructional ModelAliaa IryaniNessuna valutazione finora
- Design-Based Research in Science Education: One Step Towards MethodologyDocumento15 pagineDesign-Based Research in Science Education: One Step Towards MethodologyjuliaNessuna valutazione finora
- IN120085064 Chemistry TeacherLabManual 2013 Ch2Documento10 pagineIN120085064 Chemistry TeacherLabManual 2013 Ch2Resta RatnaNessuna valutazione finora
- Physics Syllabus Outline:: Higher Level (240 Hours)Documento2 paginePhysics Syllabus Outline:: Higher Level (240 Hours)Hari SudevvaNessuna valutazione finora
- Makalah Scientific ApproachDocumento8 pagineMakalah Scientific ApproachREZANessuna valutazione finora
- The Levels of Inquiry Model of Science Teaching-TerjemahanDocumento18 pagineThe Levels of Inquiry Model of Science Teaching-TerjemahanYesinta Mikana0% (1)
- Levels of InquiryDocumento14 pagineLevels of InquiryTelon_catsNessuna valutazione finora
- Laboratory and Discussion Method of TeachingDocumento18 pagineLaboratory and Discussion Method of TeachingEmmanuel OrimogunjeNessuna valutazione finora
- DIK 18A Intan Suhariani 218112100 CJR Research MethodologyDocumento9 pagineDIK 18A Intan Suhariani 218112100 CJR Research MethodologyFadli RafiNessuna valutazione finora
- Thesis Chapter 1Documento10 pagineThesis Chapter 1Maria Camille Villanueva SantiagoNessuna valutazione finora
- Boosting Science Skills with Inquiry LabsDocumento35 pagineBoosting Science Skills with Inquiry LabsAngelou CarlosNessuna valutazione finora
- The Laboratory in Science Education: Foundations For The Twenty-First CenturyDocumento27 pagineThe Laboratory in Science Education: Foundations For The Twenty-First CenturyIjaz AhmadNessuna valutazione finora
- The Laboratory in Higher Science Education: Problems, Premises and ObjectivesDocumento18 pagineThe Laboratory in Higher Science Education: Problems, Premises and ObjectivestahirNessuna valutazione finora
- Students Integrate Knowledge Acquisition and Practical Work in The LaboratoryDocumento5 pagineStudents Integrate Knowledge Acquisition and Practical Work in The LaboratorySinisa RisticNessuna valutazione finora
- METLIT.1.Characterizing Educational Design ResearchDocumento27 pagineMETLIT.1.Characterizing Educational Design ResearchWhindi Widhiyanti SNessuna valutazione finora
- Online Science Readings Chapter 1: What Is Science Inquiry?Documento171 pagineOnline Science Readings Chapter 1: What Is Science Inquiry?RebScrbdNessuna valutazione finora
- Investigative Primary ScienceDocumento23 pagineInvestigative Primary ScienceTiago Fernando Alves de MouraNessuna valutazione finora
- Mscied BiologyDocumento4 pagineMscied BiologySein Borongan VillanuevaNessuna valutazione finora
- Constructive Action Research A Perspective On The Process of LearningDocumento19 pagineConstructive Action Research A Perspective On The Process of LearningGizzelle LigutomNessuna valutazione finora
- 25 Years of Inquiry-Oriented Learning in LaboratoriesDocumento9 pagine25 Years of Inquiry-Oriented Learning in LaboratoriesWiwikKartikaSariNessuna valutazione finora
- Sci WsDocumento26 pagineSci WsAndrewNessuna valutazione finora
- Sampson2010 PDFDocumento41 pagineSampson2010 PDFAriny ZaqiyahNessuna valutazione finora
- Curry Action ResearchDocumento4 pagineCurry Action ResearchDanielGonzálezZambranoNessuna valutazione finora
- Implementing cooperative learning to improve Life Science concept understandingDocumento7 pagineImplementing cooperative learning to improve Life Science concept understandingKarabo SelepeNessuna valutazione finora
- David NunanDocumento15 pagineDavid NunanTrinh Anh MinhNessuna valutazione finora
- (Ita) Seeing Is BelievingDocumento5 pagine(Ita) Seeing Is BelievingNiken SweetNessuna valutazione finora
- The Learning CycleDocumento25 pagineThe Learning Cyclehanafi5527Nessuna valutazione finora
- MetapedagogyDocumento12 pagineMetapedagogyThe AllianceNessuna valutazione finora
- International Journal of Educational Research: Xenia MeyerDocumento15 pagineInternational Journal of Educational Research: Xenia MeyerNaveen NaveenNessuna valutazione finora
- Faculty of Education and Languages (SMP) : Teaching Science For Upper Promary IiDocumento27 pagineFaculty of Education and Languages (SMP) : Teaching Science For Upper Promary IiSri GanggaNessuna valutazione finora
- Effects of Active-Learning Experiences On Achievement, Attitudes, and Behaviors in High School BiologyDocumento20 pagineEffects of Active-Learning Experiences On Achievement, Attitudes, and Behaviors in High School BiologyTaregh KaramiNessuna valutazione finora
- I 1.1. The Inquiry Laboratory in Science EducationDocumento16 pagineI 1.1. The Inquiry Laboratory in Science EducationTavia BrinleyNessuna valutazione finora
- Core Set PDFDocumento37 pagineCore Set PDFDana TaladroNessuna valutazione finora
- Values in The Science Classroom - The Enacted' Curriculum: Mary RatcliffeDocumento18 pagineValues in The Science Classroom - The Enacted' Curriculum: Mary RatcliffeAizel NaranjoNessuna valutazione finora
- The Laboratory in Science Education: Foundations For The Twenty-First CenturyDocumento27 pagineThe Laboratory in Science Education: Foundations For The Twenty-First CenturyKoimahNessuna valutazione finora
- Sampson 2013Documento28 pagineSampson 2013Dea PermataNessuna valutazione finora
- Teaching Science Using the Inquiry ApproachDocumento14 pagineTeaching Science Using the Inquiry ApproachAtarah Elisha Indico-SeñirNessuna valutazione finora
- 5 E Instructional ModelDocumento10 pagine5 E Instructional ModelRenzdy MejillaNessuna valutazione finora
- Research Project B.Ed (1.5 Year/2.5 Year) Code No: 8613: by DR - Tariq Mehmud YousafzaiDocumento14 pagineResearch Project B.Ed (1.5 Year/2.5 Year) Code No: 8613: by DR - Tariq Mehmud YousafzaiIqra MirzaNessuna valutazione finora
- ConstructivistDocumento4 pagineConstructivistjukukoNessuna valutazione finora
- TSCIEDocumento7 pagineTSCIEMichael VillanuevaNessuna valutazione finora
- Uncovering Student Ideas in Science, Volume 3: Another 25 Formative Assessment ProbesDa EverandUncovering Student Ideas in Science, Volume 3: Another 25 Formative Assessment ProbesNessuna valutazione finora
- Uncovering Student Ideas in Science, Volume 4: 25 New Formative Assessment ProbesDa EverandUncovering Student Ideas in Science, Volume 4: 25 New Formative Assessment ProbesNessuna valutazione finora
- DigesDocumento4 pagineDigesFehmeed AlchemyNessuna valutazione finora
- WSGDocumento2 pagineWSGFehmeed AlchemyNessuna valutazione finora
- FACT: Different Kinds of Microbes Cause Different DiseasesDocumento1 paginaFACT: Different Kinds of Microbes Cause Different DiseasesFehmeed AlchemyNessuna valutazione finora
- Waterborne Diseases: Symptoms and CausesDocumento1 paginaWaterborne Diseases: Symptoms and CausesFehmeed AlchemyNessuna valutazione finora
- PGCE Secondary Science Handbook 2009-2010 Final DraftDocumento68 paginePGCE Secondary Science Handbook 2009-2010 Final DraftFehmeed AlchemyNessuna valutazione finora
- IB HL SL Ma Thematic Sample QuestionsDocumento78 pagineIB HL SL Ma Thematic Sample QuestionsNamrata Batra100% (1)
- Grade 12 Chemistry GED Homework 4 Aldehyde & Ketones Name: - Mark: - /10Documento2 pagineGrade 12 Chemistry GED Homework 4 Aldehyde & Ketones Name: - Mark: - /10Fehmeed AlchemyNessuna valutazione finora
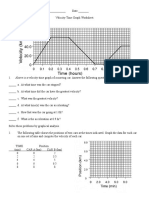
- Velocity-Time Graph WorksheetDocumento2 pagineVelocity-Time Graph WorksheetFehmeed AlchemyNessuna valutazione finora
- Food and DigestionDocumento120 pagineFood and DigestionFehmeed Alchemy100% (1)
- Polo Pol PolopoloDocumento20 paginePolo Pol PolopoloFehmeed AlchemyNessuna valutazione finora
- 9709 w12 QP 32 PDFDocumento4 pagine9709 w12 QP 32 PDFAj AgenNessuna valutazione finora
- Downward - 2Documento9 pagineDownward - 2Fehmeed AlchemyNessuna valutazione finora
- States of Matter PDFDocumento4 pagineStates of Matter PDFFehmeed AlchemyNessuna valutazione finora
- Some Common Pollutants: Air Pollution: Harmful Substances in The AirDocumento3 pagineSome Common Pollutants: Air Pollution: Harmful Substances in The AirFehmeed AlchemyNessuna valutazione finora
- ElectrostaticsDocumento18 pagineElectrostaticsFehmeed Alchemy100% (1)
- SAT II Physics Formula SheetDocumento10 pagineSAT II Physics Formula Sheetalex100% (1)
- University of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelDocumento12 pagineUniversity of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelFehmeed AlchemyNessuna valutazione finora
- Moles IIDocumento2 pagineMoles IIRylieJaneNessuna valutazione finora
- RedistDocumento1 paginaRedistJeve MilitanteNessuna valutazione finora
- Properties of Chemical Bonds KEYDocumento1 paginaProperties of Chemical Bonds KEYFehmeed AlchemyNessuna valutazione finora
- Paper 1 Chemistry SPM SBPDocumento19 paginePaper 1 Chemistry SPM SBPsizzledeedle100% (2)
- Moles Worksheet: Calculate Amounts of Chemical SubstancesDocumento1 paginaMoles Worksheet: Calculate Amounts of Chemical SubstancesFehmeed AlchemyNessuna valutazione finora
- BalancingampChemical EquationsDocumento21 pagineBalancingampChemical EquationsFehmeed AlchemyNessuna valutazione finora
- Properties of Chemical Bonds KEYDocumento1 paginaProperties of Chemical Bonds KEYFehmeed AlchemyNessuna valutazione finora
- RLDocumento1 paginaRLFehmeed AlchemyNessuna valutazione finora
- Moles Worksheet: Calculate Amounts of Chemical SubstancesDocumento1 paginaMoles Worksheet: Calculate Amounts of Chemical SubstancesFehmeed AlchemyNessuna valutazione finora
- POGIL 01 - Nomenclature 1 - IonsDocumento2 paginePOGIL 01 - Nomenclature 1 - IonsFehmeed AlchemyNessuna valutazione finora
- Understanding Alien Culture Through Limited InformationDocumento2 pagineUnderstanding Alien Culture Through Limited InformationFehmeed AlchemyNessuna valutazione finora
- Journal of Statistical Planning and Inference: Akanksha S. KashikarDocumento12 pagineJournal of Statistical Planning and Inference: Akanksha S. KashikarAkanksha KashikarNessuna valutazione finora
- Reservoir Characterization 3 LoggingDocumento47 pagineReservoir Characterization 3 LoggingMohamed AbdallahiNessuna valutazione finora
- Amity International Business School Mba Ib: Production & Operations ManagementDocumento12 pagineAmity International Business School Mba Ib: Production & Operations ManagementSHIVAM JAINNessuna valutazione finora
- Soft Start - Altistart 48 - VX4G481Documento2 pagineSoft Start - Altistart 48 - VX4G481the hawakNessuna valutazione finora
- Chapter 1Documento9 pagineChapter 1Ibrahim A. MistrahNessuna valutazione finora
- Guia de Manejo Sdra 2019Documento27 pagineGuia de Manejo Sdra 2019Jorge VidalNessuna valutazione finora
- Herschel 10027757Documento83 pagineHerschel 10027757jurebieNessuna valutazione finora
- Soal Biokim IDocumento9 pagineSoal Biokim INuraMalahayatiNessuna valutazione finora
- 11 - Chapter 5 PDFDocumento35 pagine11 - Chapter 5 PDFlouisNessuna valutazione finora
- Friction Stir Welding: Principle of OperationDocumento12 pagineFriction Stir Welding: Principle of OperationvarmaprasadNessuna valutazione finora
- Trigonometry Ted Sundstrom and Steven SchlickerDocumento430 pagineTrigonometry Ted Sundstrom and Steven SchlickerhibiskusologjiaNessuna valutazione finora
- 2 Reason Why I Like DoraemonDocumento2 pagine2 Reason Why I Like Doraemonpriyanka shafiraNessuna valutazione finora
- Introduction To Literary TheoryDocumento3 pagineIntroduction To Literary TheoryAnil Pinto100% (4)
- Cloud Computing Vs Traditional ITDocumento20 pagineCloud Computing Vs Traditional ITgarata_java100% (1)
- "Difference Between Private and Public Nuisance": Law of TortsDocumento4 pagine"Difference Between Private and Public Nuisance": Law of Tortsaridaman raghuvanshiNessuna valutazione finora
- Citizen Journalism Practice in Nigeria: Trends, Concerns, and BelievabilityDocumento30 pagineCitizen Journalism Practice in Nigeria: Trends, Concerns, and BelievabilityJonathan Bishop100% (3)
- Week3 Communication Skill Part 1 Student GuideDocumento10 pagineWeek3 Communication Skill Part 1 Student GuideZoe FormosoNessuna valutazione finora
- Political Philosophy of J S MillDocumento9 paginePolitical Philosophy of J S MillRajkumar SunnyNessuna valutazione finora
- SINGLE OPTION CORRECT ACCELERATIONDocumento5 pagineSINGLE OPTION CORRECT ACCELERATIONShiva Ram Prasad PulagamNessuna valutazione finora
- IT Workload Types: Static, Periodic, Once-in-a-lifetime, Unpredictable, Continuously ChangingDocumento3 pagineIT Workload Types: Static, Periodic, Once-in-a-lifetime, Unpredictable, Continuously ChangingAnand KumarNessuna valutazione finora
- Vblock® Systems Password ManagementDocumento22 pagineVblock® Systems Password ManagementVakul BhattNessuna valutazione finora
- Difference Between Defect, Error, Bug, Failure and FaultDocumento28 pagineDifference Between Defect, Error, Bug, Failure and FaultbhojanNessuna valutazione finora
- Chapter 1&2 Exercise Ce StatisticDocumento19 pagineChapter 1&2 Exercise Ce StatisticSky FireNessuna valutazione finora
- Professional Training Academy: Professional Level IV - Preparation & Final TestDocumento2 pagineProfessional Training Academy: Professional Level IV - Preparation & Final TestJayant SinhaNessuna valutazione finora
- Devki N Bhatt01240739754Documento10 pagineDevki N Bhatt01240739754menuselectNessuna valutazione finora
- Handbook Fof Social MobilizationDocumento32 pagineHandbook Fof Social MobilizationAnith BaylisNessuna valutazione finora
- Cbcs Syllabus 2016-17 Admn Batch 30.07.16Documento23 pagineCbcs Syllabus 2016-17 Admn Batch 30.07.16Sushree sonali NayakNessuna valutazione finora