Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Teach Quantum Mechanics To Your Dog by Ryan Lott (2012)
Caricato da
ryanlottDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Teach Quantum Mechanics To Your Dog by Ryan Lott (2012)
Caricato da
ryanlottCopyright:
Formati disponibili
Teach Quantum mechanics to your dog
by: Ryan Lott
2012
Introduction I have always loved science, physics in particular, with a passion ever since I was a child. Something about it always just drove me crazy and ever since, I've had a thirst for its knowledge. Recently, I started an in-depth of study quantum mechanics after hearing all the fuss about it. I always heard that it would absolutely blow my mind and change my entire perspective on not only life but also what I believe in and how I think. I always thought that this was silly and therefore never considered the ideas of it literally transforming people. Boy, was I wrong. It taught me that there are limits on human knowledge and that, without a doubt, something more interesting than what the eye sees is going on. My gift is science, and through that I will attempt to give you a taste of greater truth. In this report, I will give you a solid introduction of quantum mechanics, using almost no math, simple language, and pictures on every page for entertainment, cats included! I worked hard to try to make any person understand the basics of quantum mechanics for the love of science.
Enjoy.
BEFORE YOU START READING: WATCH THIS VIDEO:
http://www.youtube.com/watch?v=DfPeprQ7oGc
IT IS VERY CRUCIAL TO THE REPORT.
"You are not meant to be comfortable with the conclusions of quantum mechanics." - Al-Khalili
What is fate? A lot of people enjoy the idea of being able to predict the future with great certainty. For instance, many humans partake in the act of horoscopes, gazing up at the orientations of stars and constellations to determine their physical future. What about throwing a double-sided coin that always shows heads when you throw it? Obviously, you can predict that the coin, no matter how high you toss it, when, or where, that it will always show heads. What about something more complicated? Take the weather for instance. Everyone knows that weathermen might say that it will be sunny skies the next day, but actually end up raining like cats and dogs. This process may sound so complex and random that you say, "It's impossible to predict the weather correctly," but at the same time it makes complete logical sense to think that if the weatherman knew everything about the system of the world, that he could predict, without a doubt, what the weather would be the next day, perfectly. For a very long time up until the early 20th century, many (almost all physicists) believed in this principal, including Isaac Newton, the father of the fundamental universal laws of motion. Determinism or Clockwork Universe is just this; the idea that the future can be completely determined if we have full knowledge of the present. If I know the starting point of an object, it's speed, direction ( speed + direction = velocity ), mass, and all of its surrounding forces and obstacles, I can predict the absolute future using Newton's laws; how they glide, collide, swoop, and swerve, no matter how complex the situation. Of course, in reality, this is completely impossible except for the most simple systems and situations.
We all know that weathermen can't always confidently predict tomorrow's weather, and that we can't predict whether a normal coin will land heads or tails. Chaos Theory is studied by many people in modern days, which states that in order to completely predict the future, we would have to know all of the initial conditions of a system to infinite accuracy. I'm sure many of you have heard of the 'butterfly effect'. If a butterfly were to flap its wings, it could move particles, which would move more particles, which would move more particles, and so on and so on, ultimately changing and affecting the future of the world, possibly causing a great thunder storm on the other side of the world fifty years from when it flapped its wings. This is a very provoking and valid thought in Chaos Theory. We will come back to this idea later. I don't want to confuse you with different logical theories of the universe, so let's just stick with determinism for now. Even sadder, you could easily say that the human brain is made up of atoms, and so all of our thoughts, feelings, and actions are nothing more than an interaction of particles and atoms following Newton's laws of motion. This is a rather depressing viewpoint of life, but it is a legitimate thought. Fortunately, human logic has been completely transformed by quantum mechanics. The very concept and existence of quantum mechanics utterly destroys the entire idea of determinism, and brings forth indeterminism. So to you who believe that we live in a clock work universe, turn away now, because we have stumbled into a new realm; a new reality of truth. The world of Quantum Mechanics. Billiards, anyone? Imagine a game of pool. You rack up the balls, set up the cue ball, break, and then continue to perform legal actions of the game until a winner claims victory. Let's say you recorded the entire game to insane detail with a camera, and you want to tell a computer to recreate an exact replica of the game you just played. How would you do this? Well, you know that for every shot, every ball will go a certain direction, with a certain speed, and collide with the other balls and
barriers in a certain way. So you think, "This is easy, I'll just program the computer to know exactly how hard I hit the cue ball, and the exact angle at which I struck the first ball I was aiming at!" Sounds simple enough, but is this really all the information you would need? What if some of the balls were microscopically potted into the table, just like a marble would be potted if you were to drop it into sand? It's easy to predict what would happen if only two balls were to collide during one shot, but how complicated would it be if multiple balls were collided and scattered in one shot? If one moves just a tad bit different than expected, an entirely different outcome would be produced. So now, not only do we have to know where the cue ball and the first ball struck are, during each shot, at all times, but ALL the balls' positions on the table. Now things are getting really complicated. Even further, what would happen if a tiny piece of dust floating through the air were to land on one of the balls; surely there is some effect on the final outcome of the shot. Does this remind you of anything? (the 'butterfly effect') Temperature, humidity, and even radiation from the light in the room could be effecting the entire outcome of every shot, and the game as a whole. This may seem somewhat baffling, but this is the reality of the situation. But then you say to yourself, "Okay, so it's hard to exactly model a game of pool, but it's not impossible! Yes, there is only a probability to a certain degree of action for each shot, but I'm just lacking information to create an exact replica of the game. Aha! I've defeated you, science!" So now you come to a conclusion: the more you know about the system, the more you can accurately predict the future, no matter what the situation. The same would be if you were tossing a coin in the air. Let's say you flip a coin and it lands heads. You could, in theory, model the exact same coin flip. But is this practical? No, but the bottom line is that there is the possibility of achieving the same identical outcome. This is what Newtonian determinism is all about. Knowing the full initial conditions of a systems, you can completely recreate an entire event, in a deterministic behavior. This is also called Classical Mechanics; the norm for all objects
in the universe whether it is a game of soccer, the planets orbiting the sun, or your grandmother's dentures falling out of her mouth. However, in the subatomic quantum world, things are very different. Unpredictability in the Quantum World In the quantum world, unpredictability is not only an option, it is a certainty; one that doesn't result from a lack of information, but because of nature itself. The reality of the subject is that all we can do is nothing more than calculate probabilites of a given subatomic system for a particular outcome. But this isn't your average intuitive probability, such as the probabability of a coin landing on heads (which is .5). No, quantum probabilites are actually built into the theory itself, and we can't do any better than that, I'm sorry to say. Conside radioactive decay. Most students have heard of the concept of half-life: the time required for something to fall to half its initial value (in particular, the time for half the atoms in a radioactive substance to disintegrate). Say you have 1 million atoms. The half-life would be the time it takes for 500,000 of those 1 million atoms to disintegrate. Have you ever heard of carbon dating? Half-life is one of the main contributors to this phenomenon. Half-life is only meaningful when you have a statiscally large number of atoms, such a the 1 million stated above. There is no formula (or way) to tell when a single nucleus will decay. All we can do, through quantum mechanics, is predict the probability of a nucleus decaying, nothing more. The important thing to see here is that this is not due to the lack of the information we have of the atom, but because of the fundamentals of quantum mechanics itself. One could say, "Okay, well then quantum mechanics doesn't show the entire picture of what's going on." Einstein held this belief when he said that 'God does not play dice'. Just like the possibilites of recreating the pool game or the coin toss that we dicussed earlier, the principle of atomic decay should be no different. If this were true, then we would have to go further than quantum mechanics. In the end, this
theory is incorrect. I will discuss this in another paper. But for now, it is fact that quantum mechanics suggests that our world, as we know it, is, under the hood, isn't absolutely predictable. Along came Schrdinger Earlier in the report, we discussed Newtonian determinism. For large objects, like golf balls, people, and even drones blasted into space by NASA, the all rely on Newton's laws. And they should! They're completely accurate for such objects! So why is it that we can't describe something like an electron or an atom with Newton's equations if they work for other things in real life? It turns out that scientists have discovered that this is just a plain fact of nature and you must accept it.
A lot of the credit for the mathematical and theoretical aspects of quantum mechanics belongs to a man named Erwin Schrdinger. If it were not for his existence, your computers, iPhones, televisions, or any electronic device ever made would most likely NEVER exist. Physicists, specifically de Broglie, had already found out that matter has wave like behavior and needed a way to mathematically explain these properties.(Look up on the famous YouTube webpage for more info on de Broglie waves) That's where Schrdinger came in. He proposed an equation so great that it would explain many phenomena in the world and would result in a technological evolution. It's called Schrdinger's Equation, an equation that describes NOT how a particle moves but the way a wave evolves over time in space (since particles and waves are related, this equation is useful). It's called a wave equation. The result of this equation is the Wavefunction (a mathematical quantity describing a wave in a system). A breathtaking discovery at that. I will not go into the mathematics of the equation, but I will tell you that is called reduced-Planck's constant, is called the Laplacian Operator (how fast the wave changes over space, comparable to a car
accelerating and decelerating), m is the mass of the particle, (pronounced 'Psi') is the wavefunction itself (what we desire to obtain by solving the equation), i is called an imaginary number (although they
are very real), and
is called the 'partial time derivative' of the wavefunction, which is how the
wavefunction changes with time. Solving the equation shown on the last page, as stated, will give us a mathematical quantity called the wavefunction. This result tells us ALL the information that we have to predict the future of a particle, such as an electron, in a particular environment with particular initial conditions. When I say initial conditions, I mean the starting qualities of a system. For example, if I drop a ball from 10 feet in the air, it's initial conditions are that it is 1) 10 feet in the air and 2) At rest (not moving) while I'm holding it in the air before I drop it and gravity takes over. From this, we can calculate the probability of, let's say, an electron "doing this, or being here, or going there, etc." It truly is a beautiful result. I always thought it would be cool if the genie from Aladdin were in control of what an electron does next in a system, since the word 'magic' is basically one of the best ways to describe the wavefunction. A Wave or a Particle? I will try to make this section as simple as possible. Please bear with me. Pretend that you're blindfolded and I lay a marble on a table. Also, pretend I have the ability to freeze time on the table. You don't know where it is. I 'freeze' time on the table, you remove your blindfold and then look at the table. You say, "I know exactly where the marble is!" Okay, that's great. But then I ask you, "Alright, smarty pants, now where did it come from and where is it going?" You could easily say that I put it in that particular spot, but this isn't the point. You really don't know whether it was sitting still on the table the entire time, moving left, right, up, or down. There is no way for you to know. Conclusion: you know exactly where the marble is ( 'localized' position), but don't know where it came from or where it's going ('delocalized' momentum).
Now let's look at travelling waves, like tides in the ocean. If you don't know about travelling waves, watch this video on YouTube (http://www.youtube.com/watch?v=luXW6AFSloA specifically pay attention to periodic waves and frequency in the video). If you were to look at a single periodic wave, such as the one shown on the right, you could say, "I know the frequency of the wave, so I can tell what it's energy and momentum is!" (By the way, momentum is how much 'oomph' a wave or piece of matter has for those of you who don't know) You just might win the next noble prize, until I ask, "Okay, where is the wave?" Woah. What? Is that even a legitimate question? You say, "Well no! I mean... it's a wave! It's spread out over space... right...?" Exactly! You win! It doesn't make sense to ask where is the wave, so therefore it's plainly obvious that we don't know where the wave is. Conclusion: you know the frequency of the wave therefore you can know where it's going, how fast, and how much energy it's carrying ('localized' momentum), but you have no clue where the wave really is ( 'delocalized' position). So where am I getting at, you ask? Well, in quantum mechanics, matter can behave like particles (marbles) in one instance, and waves in another instance. WHAT?!? How can this be? It's incredibly unintuitive, but the answer is simply that this is how quantum mechanics works. Remember the quote at the top of the first paragraph? Exactly. I am not saying that matter is both a wave and a particle (marble) at the same time, nor are they only one or the other, but that these properties are fundamentally built in to the theory of quantum mechanics itself. There is no escaping this fact. In reality, the scary truth is that we have no clue what is going on. We can only say that atoms, electrons, and other subatomic particles that make up our universe exhibit both behaviors in different instances, and we can only calculate probabilities based on these principals; I'm sorry to disappoint you. Keep these conclusions closely by, for you will need them later on in the report.
Electron Trapped in a Box Imagine you have a box and in this box there is a 'floating' marble. (it can be anywhere in the box). Common sense will tell you that the marble is only in one spot at a particular time. This is correct. Simple enough. Now, let's pretend that for a moment the marble is replaced with an electron. By... 1) Assuming I know where it is in the box at the start of the experiment 2) The dimensions of the box 3) All the forces and obstacles in the box 4) The initial energy of the electron ... I can calculate the value of the wavefunction using the Schrdinger's equation for every point in space in the box. So why is this important? Well, if I know all these values at every point, then I can predict where the electron will be in the box! The probability is different for different locations, therefore the possibility of where it is is spread out, hence the term 'wavefunction'. Clever, huh? Not so hard now is it? Physics can be easy! It is important for me to say that no one really knows what the wavefunction is. You can't measure it, nor observe it. A lot of people say it is actually real, whereas some say it's just a math quantity. There is great debate on this subject, but to make our lives simple, let's just all agree that it's just a mathematical quantity that we can use to predict the future. So we have the values of the wavefunction for this situation. Now what? How do we do all this predicting that we've been talking about? Well, every value for every point is called a complex number. I will not go into detail on this but will provide a diagram to the right to help demonstrate what I mean. Basically, each value is assigned two numbers. One 'real' (a), one 'imaginary' (b). If you know some mathematics, using the picture on the right and by using the Pythagorean theorem, we can find the magnitude (or modulus for
you math nerds) of this complex number. Remember that each value for each point in space has its own complex number. Now if we take the square of the magnitude, (also said as the magnitude x the
magnitude), we now obtain the probability of finding the electron at that point! Do this for ALL points in the box, and you have just obtained the probability density distribution of where the electron will be in the box! Astonishing! To make things simple, we assign colors to these values. As for the picture shown on the previous page, the color of choice is green and the brighter the 'color point' is, the higher the probability of the electron being there is! So for this picture, it looks like the electron will probably be in the left hand side of the box. In general, with this technique, we can calculate the probability of actions for ALL POSSIBLE situations in the universe. Because of this, you can read these words from your computer monitor, iPhone, etc. It truly is a remarkable invention of mankind and the best part is that it WORKS. Crime Waves We just discussed in the previous section how the wavefunction works, and how we can calculate the probabilities of particles 'doing this' or 'doing that'. But we're forgetting something. How does the wavefunction change with time? After all, things in the universe don't stay static, such as the changing of weather patterns. Now, it's fairly difficult to explain this concept in regards to the wavefunction with scientific language, so I will provide you with an analogy. Suppose you are a police man, and you just received a report of a bank robbery. You know where the burglar was located at the start of the crime (the bank). So you know that there is a high probability that he must be in the area near the bank. Once you get to the bank, you talk to witnesses in the area and receive clues about the direction the bank robber was heading after the crime. Maybe someone said they saw him get in a car and head downtown. This changes your intuition and the
probabilities of where the bank robber is. You now know there is a higher probability of him being downtown. You continuously follow clues, with the probabilities of his location in various spots of the city constantly changing, until you ultimately, and hopefully, detect and arrest the criminal for the dirty deeds he has committed. So basically, the clues of where the robber is heading, scientifically, changes your initial conditions, and thus the 'criminal wavefunction' overall. Make sense? Replace the criminal with the electron and you have just solved the case, in regards to physics. If an electron is discovered or hinted at being in a particular spot, the wavefunction instantly changes, and evolves overtime. But there is a difference between the robber and an electron. A huge difference. The bank robber can only be at one place at one time (obviously), but the electron, doesn't exist as a simple particle, or object, with a specific location at a point in time. It's influence is spread out all over space, and the worst part is, I'm sorry to break to you, that we don't know why this is so. All we have to describe the electron is the wavefunction and, as demonstrated in the video, as soon as we look to see what it's doing, it's behavior changes and the wavefunction collapses, and goes back to behaving like a particle. It is, in my opinion, the 8th wonder of the world, and the most magical one at that. I like to call it God's Conundrum. So that's it? If you're not disturbed by everything you've read and learned so far, you might want to take a step back and realize what you're dealing with here. You might think that this possibly can't be all to it. This can't be the end. Just because we don't know exactly what it's doing, doesn't mean it isn't doing something. Einstein thought the same way, but physicists have pretty much thrown in the towel and accepted what they have discovered. However, there are theories out there from small groups that claim that there could be an answer. This, unfortunately, will need to be described in another paper. But if you
have made it this far without a decaying interest, I applaud you. Because now the really freaky and intriguing stuff starts to begin. Who the Heck is Heisenberg? We've learned from quantum mechanics, that just because we know the initial conditions of a system and have information about that system doesn't mean that we know exactly what it's going to do. We dubbed this indeterminism. Not only does quantum mechanics exhibit indeterminism, but also something far more stressful; indeterminacy. This states that we can never simultaneously know everything about a system at a particular instance in time, not even if we try to measure everything all at once, such as position, momentum, energy, and so on. How? How could there be such a limit to just MEASURING? Recall what we learned about particles and waves and their localized and delocalized position and momentum properties. Now ask yourself a question: "Is the electron being like a particle or a wave(spread out over space)? And if it's behaving like one or the other, what does this say about it position and velocity?" Well, a very intelligent man by the name Werner Heisenberg came up with what is known as Heisenberg's Uncertainty Principle. Now, it takes a lot of advanced mathematics to explain what this concept says, but I'm going to make it easy for you and give you the gist of the matter. It turns out that from the nature of quantum mechanics, it is impossible for someone to precisely know the position and the velocity (or momentum) of an electron or particle in a system simultaneously. So far, we've been discussing the 'position wavefunction' in this paper, but there are other wavefunctions as well, in particular, the 'momentum wavefunction'. However if you know one wavefunction, you can do some crazy math (Fourier Transform) to yield the other wavefunction and vice versa. The bottom line here is that, because wavefunctions tell us everything we can possibly know about a particle in a certain state ("doing this or that"), and because of the uncertainty principle, we are limited about what we can know about a system at one time. To make easier sense of this, look at the picture on the left. It can be two faces or a vase, but not both at the same time. This principal is applied to particles as well. Creepy.
Whew, that was a lot. Wait, there's MORE? As I said earlier if you're not surprised by the conclusions of what quantum mechanics brings forth then you need to watch the video and read the contents of this report up until now again. If you're in disbelief and think that this is tomfoolery then congratulations, you have just taken your first step. Obviously what we are dealing with here makes absolutely no sense, and I personally believe God made it that way on purpose. But for now let's recap. We learned that the wavefunction helps us predict what a particle is doing in a specific environment. Remember, the wavefunction only predicts what the particles, atoms, and electrons are doing. In no way am I saying that the wavefunction is a description of what the particles are or that, even worse, that it is the particle itself. We have no clue of what's actually going on. Obviously, quantum mechanics is a very disturbing and strange realm, and the amount of applications it has to current science methods, phenomena, and wacky theories is infinite. The really is no intuitive way of explain it. It just is. And so now I would like to return back to what's going on in the video and give some insight. Now we jump back into the fun side of the story (although I think all the math and theory is exhilarating). Super WHAT?! I'm sure most of you have cast a pebble into a calm pond. You've seen how it hits the water and one ripple (or water wave) smoothly progresses across the pond. A beautiful sight at that. Now we've all gone to the beach or a pool or even a bath tub. People are splashing in the pool, multiple tides are crashing at once, and your rubber ducky is wobbling around like it has spaghetti legs. What's causing this? The combination of all the disturbances in the water are the culprit! When one water wave (or waves
in general) meet another, they add up! Sometimes when two waves hit, they can make a bigger wave, or they can even cancel each other out. Do this over a million times in one pool and you have a really wavy pool. This combination of waves is called Superposition, and not only does it apply to things like pools and the ocean, but quantum mechanics too! Just for reference and insight, say that you do the exact same experiment that they did in the video but let's say that they did it with light instead of electrons. What would you see? Well, light behaves like a wave and so due to superposition and also diffraction(look up on YouTube, for I'm far to puzzled at how to explain this principle), if a light beam were to hit and go through the two slits, it would also create an interference pattern on the back wall just like the water waves in the video did! So, what's going on with the electron when it hits the slits? How does it make the interference pattern? In order to even remotely explain this, we need a wave-like structure, so physicists apply this to the electron. Remember that all we have is the wavefunction. This is the wave-like structure that we need! Let's treat the electron like a water wave(really, a wave in general). And here's the best part. Just like water or light waves, the rules of superposition apply to the wavefunction! HOORAY! The fact that this is possible is the only thing, I believe, that allows physicist to sleep at night. Okay, so superposition applies to the wavefunction. Big deal. "What's the point?" you ask. Bear with me on the next bit of information, for it is very difficult to grasp. Imagine an electron travelling down a path at a certain speed. It has a particular wavefunction. We'll call it WF#1. Now, imagine that the exact same electron decides to slow down. Obviously it's journey will be altered. The original wavefunction is altered and produces WF#2. Now, because of superposition, it's possible for the electron
to exist in a state modeled by a third wave function (WF#1 + WF#2 = WF#3) What does this mean? It means the electron can be in a state where it is moving fast AND slow AT THE EXACT SAME TIME. WHAT?! That doesn't make logical sense! The electron is matter and the burglar is matter, and the burglar can only be in one place at a time but not the electron?! I see where you might fall into disbelief. You think, "Well the wavefunction isn't actually the electron and, after all, whenever we look to find out where the electron is, it is only in one place! Not to mention that when we measure the energy of an electron we only receive ONE value. Maybe it's a statistics thing. I don't believe you. " My response is to simply explain the double-slit experiment with that logic. I don't believe you could live long enough to solve that problem. In the end, the wavefunction and superposition is the only way physicists can 'explain' the experiment. Again, we still don't know what the electron is actually doing. Poetry in Motion If you've never read Robert Frost's poem The Road Not Taken, I suggest you take a look at it. While it is a masterpiece in its own context, it's also almost a complete description of quantum mechanics, where the electron can take both roads at once. This applies greatly to the double slit experiment and it all comes down one question. What is going on with the electron when it is fired from the gun towards those two slits? I'll do my best to 'explain' it. While a purely mathematical explanation would yield the best insight, I will use that information to explain verbally what's going on in a step-by-step manner. The fired electron has its own evolving wavefunction. When it hits the two slits, the wavefunction splits into two pieces. Since the electron occupies both wavefunctions, which makeup the original, there
is an equal likelihood of it going through both slits. The two spread-out wavefunctions are travelling towards the back wall and, because of superposition, they will end up interfering with each other, just like the water waves in the video, and give us the interference pattern on the back wall. The only difference here is that instead of having a physical wave hit the wall, we are only given a probability of where the single electron hits on the back wall. And it just so happens to show up as a wavy interference pattern. That's it. This is all we have to attempt to explain the experiment. If we even try to detect which slit it actually went through, the wavefunction collapses and the interference pattern vanishes. This may seem like a depressing ending, but the positive side of all of this is that physicists can predict, with the wavefunction, many things and give you things like iPhones. Yes, again with the iPhones. But of course this doesn't satisfy you! You want the truth! After all this is just mathematics. Phooey you say. Fact is, the particle somehow stops acting like a particle, acts like a wave for a moment, then acts like a particle again to hit the screen. Pure uncensored magic. No matter how unbelievable this is, you must accept it. It is reality. No 'real' explanation has be found yet, and in my opinion there never will be, contrary to the beliefs of a very small minority of physicists, who in my opinion can't accept the truth. If you try to make logical sense of it, I don't mean to insult you but you're 1) probably wasting time and 2) desensitized to what's going on here. Although I do salute you if you decide to take a journey to discover the truth, if you dare. I do have some good news as to what's happening, although you will probably be more baffled and bewildered by what I'm going to tell you next. Physicists have created a device called an interferometer. It's basically a device where an electron is travelling down a single path and reaches a fork in the road, so to speak, and in the end, the two paths join to create one path again. It's like a GPS. You
start at the same place, and end at the same place, but can take two different routes. Each path has arms that can tell if something is going through it. Here is the scary part. From experiments, interferometers have shown that particles can actually be in superposition of two places at one. More easily said, they travel down BOTH paths at once. It would be nice to just say that the wavefunction splits just like the double-slit experiment. But the reality is that something has to be going through both arms at once. How? We don't know, only the math can explain what we see, not our eyes. We learned about the wavefunction, and how it pertains to electrons and particles, and some of the crazy things that happen in life because of the mysteries of quantum mechanics. It is here were I leave you to continue your journey in learning about the wonders of the universe. The world of quantum mechanics is a great and rewarding one. I hope this report will inspire you to journey into the rabbit hole...
2012
Potrebbero piacerti anche
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- What Is A Black Body ?Documento82 pagineWhat Is A Black Body ?Ashwani RatheeNessuna valutazione finora
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Sts Ganjil 2324 - Kimia (Responses)Documento92 pagineSts Ganjil 2324 - Kimia (Responses)HAIRUL ANAM, S.PDNessuna valutazione finora
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Timetable-Teacher Wise (Physics Department, 2021)Documento2 pagineTimetable-Teacher Wise (Physics Department, 2021)Ghulam FaridNessuna valutazione finora
- Letran de Davao, Inc.: High School DepartmentDocumento2 pagineLetran de Davao, Inc.: High School DepartmentappleNessuna valutazione finora
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Kouwenhoven - Mesoscopic e - Transport PDFDocumento680 pagineKouwenhoven - Mesoscopic e - Transport PDFThomas SatzoukidisNessuna valutazione finora
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- A Direct Calculation of The Tunnelling Current. II. Free Electron DescriptionDocumento14 pagineA Direct Calculation of The Tunnelling Current. II. Free Electron DescriptionGianlucaTirimbòNessuna valutazione finora
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Atomic Structure: ENGR45 Materials Engineering Spring 04Documento5 pagineAtomic Structure: ENGR45 Materials Engineering Spring 04KaliDasNessuna valutazione finora
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
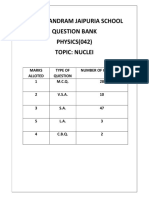
- Nuclei QB XiiDocumento23 pagineNuclei QB XiiToshani GuptaNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Inhomogeneous Electron GasDocumento8 pagineInhomogeneous Electron GasJosé Luis Rosas HuertaNessuna valutazione finora
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- IIT Jam Modern PhysicsDocumento12 pagineIIT Jam Modern Physicsshreya debnath100% (1)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Book Excerpt: 'We Have No Idea'Documento6 pagineBook Excerpt: 'We Have No Idea'Here & NowNessuna valutazione finora
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Atomic Structure: Kotz CH 7 & CH 22 (Sect 4,5)Documento37 pagineAtomic Structure: Kotz CH 7 & CH 22 (Sect 4,5)Akhmad SafrinNessuna valutazione finora
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- 0 10, Trees : Physics, The University Hew York, New YorkDocumento24 pagine0 10, Trees : Physics, The University Hew York, New YorkPrashant GokarajuNessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- Engineering Physics I Notes (All Modules)Documento159 pagineEngineering Physics I Notes (All Modules)Engineering Physics100% (2)
- 2020 YIJC Atomic Structure (Student's Copy) PDFDocumento35 pagine2020 YIJC Atomic Structure (Student's Copy) PDFLeng RyanNessuna valutazione finora
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- XRF V1 PDFDocumento83 pagineXRF V1 PDFMuhammad Robby Firmansyah100% (1)
- Schrodinger Equation: B. Quantum TransportDocumento8 pagineSchrodinger Equation: B. Quantum TransportFariba IslamNessuna valutazione finora
- Course Template Phy305 QM I New FormatDocumento3 pagineCourse Template Phy305 QM I New FormatAnuprava BokshiNessuna valutazione finora
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Periodic TrendsDocumento6 paginePeriodic TrendsOxford North100% (1)
- (UNITEXT For Physics) Lorenzo Bianchini (Auth.) - Selected Exercises in Particle and Nuclear Physics-Springer International Publishing (2018) PDFDocumento374 pagine(UNITEXT For Physics) Lorenzo Bianchini (Auth.) - Selected Exercises in Particle and Nuclear Physics-Springer International Publishing (2018) PDFSishir Jana100% (1)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- New Microsoft Word DocumentDocumento2 pagineNew Microsoft Word DocumentSwarnim KumbhareNessuna valutazione finora
- Warrior: Physical ChemistryDocumento7 pagineWarrior: Physical ChemistryGowri ShankarNessuna valutazione finora
- Assignment 1 PDFDocumento1 paginaAssignment 1 PDFHarjot SinghNessuna valutazione finora
- Coulomb BlockadeDocumento16 pagineCoulomb BlockadeSyed Mazahir Hussain RizviNessuna valutazione finora
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Atomic TheoryDocumento11 pagineAtomic TheoryRonnel GaraNessuna valutazione finora
- Noncommutative Geometry From D0-Branes in A Background B-FieldDocumento14 pagineNoncommutative Geometry From D0-Branes in A Background B-FieldmlmilleratmitNessuna valutazione finora
- Element of Quantum MechanicsDocumento32 pagineElement of Quantum MechanicsEnyuan HuNessuna valutazione finora
- QE Tutorial 9 (SpinPolarized) V8Documento14 pagineQE Tutorial 9 (SpinPolarized) V8Jose PabloNessuna valutazione finora
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Magnetic Properties and The Zeeman Effect - Chemistry LibreTextsDocumento5 pagineMagnetic Properties and The Zeeman Effect - Chemistry LibreTextsKRISHNA KUMAR GODARANessuna valutazione finora
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)