Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Giardia and Cryptosporidium Removal From Waste-Water by A Duckweed (Lemna Gibba L.) Covered Pond
Caricato da
Sandeep_AjmireDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Giardia and Cryptosporidium Removal From Waste-Water by A Duckweed (Lemna Gibba L.) Covered Pond
Caricato da
Sandeep_AjmireCopyright:
Formati disponibili
Letters in Applied Microbiology 2002, 34, 384387
Giardia and Cryptosporidium removal from waste-water by a duckweed (Lemna gibba L.) covered pond
J.A. Falabi, C.P. Gerba and M.M. Karpiscak
Department of Soil, Water and Environmental Science, Ofce of Arid Lands Studies, The University of Arizona, Tucson, AZ, USA
2001/284: received 25 September 2001 and accepted 28 January 2002
J . A . F A L A B I , C . P . G E R B A A N D M . M . K A R P I S C A K . 2002.
Aims: To determine the ability of duckweed ponds used to treat domestic waste-water to remove Giardia and Cryptosporidium. Methods and Results: The inuent and efuent of a pond covered with duckweed with a 6 day retention time was tested for Giardia cysts, Cryptosporidium oocysts, faecal coliforms and coliphage. Giardia cysts and Cryptosporidium oocysts were reduced by 98 and 89%, respectively, total coliforms by 61%, faecal coliforms by 62% and coliphage by 40%. There was a signicant correlation between the removal of Giardia cysts and Cryptospordium oocysts by the pond (P < 0001). Inuent turbidity and parasite removal were also signicantly correlated (Cryptosporidium and turbidity, P 005; Giardia and turbidity, P 001). Conclusions: The larger organisms (parasites) probably settled to the bottom of the pond, while removal of smaller bacteria and coliphages in the pond was not as effective. Signicance and Impact of the Study: Duckweed ponds may play an important role in wetland systems for reduction of Giardia and Cryptosporidium.
INTRODUCTION The ability of wetland and aquatic systems to improve the quality of waste-water is well documented (Gersberg et al. 1987; Hammer 1989; Kadlec and Knight 1996). Different studies have shown that aquatic plants, such as water hyacinth (Eichhornia crassipes L.), water lettuce (Pistia stratiotes L.) and duckweed (Lemna spp.), are capable of reducing biochemical oxygen demand (BOD), total suspended solids (TSS), and nitrogen and phosphorus concentrations in waste-water (Dewedar and Bahgat 1995). However, little or no information is available on the fate of pathogenic protozoan parasites from secondarily-treated sewage (activated sludge) applied to constructed wetlands. The removal of pathogenic organisms in aquatic or wetland systems could be the result of several factors, including natural die-off, sedimentation, predation and adsorption (Reed et al. 1995). These factors, in turn, are likely inuenced by detention time and seasonal variability. The objective of this study was to evaluate the ability of an aquatic system covered with duckweek to remove protozoan parasites (Giardia and Cryptosporidium), indicator bacteria and coliphages from activated sludge efuent. Physical-chemical data such as temperature, turbidity and pH were measured to determine whether microbial removal was related to any of these parameters. MATERIALS AND METHODS The constructed wetland facility The constructed ecosystem research facility (CERF) began operation in January 1989, and is located adjacent to the Roger Road Wastewater Treatment plant operated by Pima County in Tucson, Arizona. The facility is operated by The University of Arizonas Ofce of Arid Lands Studies for the Pima County Wastewater Management Department. The duckweed pond used in this study is 65 m long, 119 m wide and 26 m deep, with an average inuent ow rate of 55 (range 4759) litres min)1. The operational depth of the pond during this study was 09 m. The average detention time during the period of this study was estimated at 9 days. A free-oating aquatic weed called duckweed (Lemna gibba L.) was the primary plant covering the entire surface of the pond. The pond receives secondary quality efuent
2002 The Society for Applied Microbiology
Correspondence to: C.P. Gerba, Department of Soil, Water and Environmental Science, Ofce of Arid Lands Studies, The University of Arizona, Tucson, AZ 85721, USA (e-mail: gerba@ag.arizona.edu).
PARASITE REMOVAL BY DUCKWEED
385
(activated sludge) from the Pima County Roger Road Wastewater Treatment Facility diverted to CERF just prior to chlorination. Sample collection and processing Water samples of inuent (waste-water entering the pond) and efuent (waste-water exiting the pond) were collected from June 1994 through May 1996 from the duckweedcovered pond. These samples were analysed for Giardia cysts and Cryptosporidium oocysts (110 litres), total and faecal coliforms (50 ml), and coliphages (50 ml). The samples were usually collected once a month in sterile plastic bottles and transported on ice to the laboratory for analysis. Total coliform and faecal coliform bacteria samples were processed within 6 h, coliphage samples within 72 h and parasite samples within 48 h. Detection of Giardia and Cryptosporidium Volumes of inuent and efuent waste-water of 14 litres were collected directly from the pond in sterile plastic bottles. Giardia and Cryptosporidium were detected simultaneously in the samples using an indirect immunouorescence method (APHA 1992a). The immunouorescent method includes three major steps: parasite concentration into a pellet, pellet oatation to clarify the samples, and antibody staining for the detection of the parasite using a microscope with uorescent light. Giardia cysts and Cryptosporidium oocysts were concentrated from the water samples by centrifugation at 1050 g for 15 min in a swinging bucket rotor centrifuge (Jouan, Inc., Winchester, VA, USA). The pellet provided by initial centrifugation was resuspended in oated Sheathers solution (APHA 1992a). The pellet obtained, following oatation, was then distributed on pre-wetted, 25 mm diameter cellulose acetate lters with a 02 lm pore size (Costar Corp., Pleasanton, CA, USA). The lters were labelled with uorescent antibodies (Hydrouor Combo Meridian Diagnostics, Cincinnati, OH, USA). These antibodies are specic for both Giardia cysts and Cryptosporidium oocysts. During each assay, positive and negative controls ensured that assay reagents worked properly. The lters were then examined microscopically. Cysts and oocysts were identied according to specied criteria: immunouorescence, size, shape and internal morphological characteristics (APHA 1992a). The results were reported as the total number of Giardia and Cryptosporidium per litre of sample. Detection of indicator bacteria Total and faecal coliforms were detected by membrane ltration on selective media, i.e. mEndo agar (Difco) for
total coliforms and mFC agar (Difco) for faecal coliforms, according to the Standard Methods (APHA 1992b). If necessary, samples were diluted using a Tris-buffered saline solution which was prepared by mixing 1600 ml distilled water and 632 g Trizma Base (Tris hydroxymethyl amino methane; Sigma). Various dilutions of the samples were ltered through 045 lm pore size lters (Gelman Sciences, Ann Arbor, MI, USA). The lters were then placed on the selective medium and incubated for 24 h at 37C. Detection of coliphages Coliphages were detected by the double layer agar method described by Adams (1959). The host bacteria used for the assay was Escherichia coli strain ATTC 15597 (American Type Culture Collection, Rockville, MD), which detects both somatic and male specic coliphages. Waste-water samples were ltered through 022 lm pore size lters (Gelman) to remove bacteria that can interfere with the visualization of the coliphage plaques. The lters were treated with 3 ml 15% beef extract (Becton Dickinson) to avoid phage adsorption to the lters. The ltered samples (1 ml) to be assayed and 1 ml of the host strain culture were added to the previously melted soft top agar (Tryptic Soy Broth + 1% agar). All samples were assayed in triplicate. Plaque enumeration was determined after 18 h of incubation. Determination of physical/chemical parameters The inuent and efuent water turbidity was determined using a turbidimeter 2100P (Hach Company, Loveland, CO, USA). The water pH was determined using both pH indicator strips (EM Science, Gibbstown, NJ, USA) and a Corning pH meter, model 345 (Corning Inc., Corning, NY, USA). The water temperature was determined using a thermometer. Statistical analysis of the data The percentage removal of the studied micro-organisms was calculated using the following formula: % removal Ninfluent Neffluent 100=Ninfluent where Ninuent number of micro-organisms in the inuent waste-water, Nefuent number of micro-organisms in the efuent waste-water. Cyst, oocyst, faecal coliform, total coliform and coliphage percentage removals were transformed for analysis using log10(y + 1), where y number of micro-organisms. Arithmetic averages were calculated and correlation coefcients were developed for turbidity, temperature and each of the micro-organisms studied using Microsoft Excel Version 50. The average percentage removal of micro-organisms
2002 The Society for Applied Microbiology, Letters in Applied Microbiology, 34, 384387
386 J . A . F A L A B I ET AL.
was calculated using the above formula. The values of Ninuent and Nefuent used were the averaged numbers of micro-organisms in the inuent and efuent. RESULTS AND DISCUSSION During the period of this study (JulyMay), 15 sets of samples were collected for analysis approximately every 35 weeks. The average number of Giardia was 156 cysts l)1 in the inuent and 035 cysts l)1 in the efuent, resulting in an average percentage reduction of 98%. On average, Cryptosporidium oocysts decreased by 89%, with an average number of 158 oocysts l)1 in the inuent and 017 oocyst l)1 in the efuent (Table 1). Grimalson et al. (1993) studied the occurrence and removal of Cryptosporidium oocysts in Kenyan waste stabilization ponds. The oocyst levels they detected in raw waste-water samples ranged from 212 to 6213 cysts l)1. They also observed that the number of oocysts in the inuent from these ponds ranged between 3 and 230 cysts l)1. No Cryptosporidium oocysts were noted in the nal efuent from the 11 ponds studied. The minimum detention time for the removal of Cryptosporidium oocysts and Giardia cysts by the stabilization ponds was 37 days. The average number of total coliforms was 424 106 cfu (colony-forming units) 100 ml)1 in the inuent and 165 106 cfu 100 ml)1 in the efuent. The number of faecal coliforms averaged 177 106 cfu 100 ml)1 in the inuent and 597 105 cfu 100 ml)1 in the efuent. Total and faecal coliform bacteria were reduced, on average, by 61 and 62%, respectively, in the duckweed pond. This compares with the 9099% removal reported for faecal coliforms in facultative ponds with detention times of 47180 days (EPA 1983). The average number of coliphages was 1233 pfu (plaqueforming units) ml)1 in the inuent and 742 pfu ml)1 in the efuent. Coliphage reduction averaged about 40% (Table 1). Gersberg et al. (1987) found that the total number of indigenous F-specic bacteriophage (F-specic RNA and F-specic DNA phages) was reduced by about 99% after passage through a constructed wetland composed
of bulrush planted in gravel with a detention time of 55 days. They also found that poliovirus added to the wetland was reduced by 999%. During the period of this study, the temperature of the inuent to the duckweed pond ranged from 21 to 2C, and from 11 to 31C in the efuent. The turbidity values ranged from 33 to 236 NTU (Nephelometric Turbidity Unit) for the inuent and from 104 to 746 for the efuent. The turbidity removal by the pond ranged from 0 to 49%. The inuent pH ranged from 66 to 84 and the efuent pH, from 65 to 82. Pathogen removal in pond systems is believed to be due to a variety of factors, among which are temperature, natural die-off, sedimentation and adsorption (Reed et al. 1995). Helminths, Ascaris and other parasitic cysts and eggs settle to the bottom in the quiescent zone of the pond. Duckweed lacks extensive root systems onto which micro-organisms can become attached, and they also decrease sunlight below the duckweed mat; therefore, the removal of micro-organisms in duckweed-covered ponds is likely the result of sedimentation. In this study, the larger the organisms, the greater the percentage removal (Table 1). The larger organisms settle more rapidly to the bottom of the pond, while the removal of viruses was not as effective. Studies by Reed et al. (1995) on the removal of faecal coliforms and enteric viruses in multiple-cell pond systems showed a signicant reduction after passage through the pond. There was no correlation between Giardia, Cryptosporidium, coliform bacteria and coliphage removal, and the water pH and temperature. There was a weak correlation between the removal of total coliforms and Cryptosporidium oocysts (P 010). However, more data are needed to assess the signicance of that correlation. Giardia cyst and Cryptosporidium oocyst removal, and inuent turbidity, were signicantly correlated (P 001 for Giardia and turbidity; P 005 for Cryptosporidium and turbidity). The removal of Cryptosporidium oocysts and Giardia cysts was signicantly correlated (P < 0001). No correlation existed on efuent quality and the parameters studied (data not shown). Rose et al. (1991) compared the occurrence of Cryptosporidium and Giardia in surface waters and found
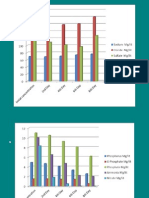
Table 1 Average densities and removal of micro-organisms by the duckweed pond Percentage removal (%) 98 89 61 62 40 Size of organisms (lm) 812 26 1115 1115 00450065
Micro-organisms Giardia (l)1) Cryptosporidium (l)1) Total coliforms (100 ml)1) Faecal coliforms (100 ml)1) Coliphages (ml)1) *Observed range of values.
Inuent 156 (429)* 158 (0325) 424 106 (822 105875 106) 177 106 (13 10553 106) 1233 (6531987)
Efuent 035 (006) 017 (005) 165 106 (85 10438 106) 597 105 (65 103122 106) 742 (691454)
2002 The Society for Applied Microbiology, Letters in Applied Microbiology, 34, 384387
PARASITE REMOVAL BY DUCKWEED
387
that the concentrations of these two parasites were signicantly correlated in all the waters analysed (P < 001). The recovery rate of Giardia and Cryptosporidium may be low when the waste-water turbidity is high because visualization of the cysts and oocysts is difcult under the microscope. There was no correlation between the removal of the parasites and the efuent turbidity. The duckweed pond was more effective in reducing the number of protozoan parasites (Giardia 98%; Cryptosporidium 89%) than indicator bacteria (total coliform 61%, faecal coliform 62%) or coliphages. The removal of microorganisms in the pond appeared to be related to the size of the organisms (Table 1). This study only assessed the physical removal of the cysts and oocysts, and not their viability. Recently, Araki et al. (2001) demonstrated that the physicochemical conditions in high-rate algal ponds were responsible for a 97% reduction in oocyst viability. Thus, the total reduction of infectious cysts and oocysts is probably greater than what was observed. Aquatic systems for waste-water treatment appear to be promising as tertiary treatment systems for enteric pathogens. Additional detention time could increase the removal capability of these systems, increasing the detention time, which would slow the ow rate through the pond, allowing more time for the micro-organisms to settle before the water reaches the outlet of the pond. REFERENCES
Adams, M.H. (1959) Bacteriophages. New York: Interscience Publisher, Inc.
APHA (1992a) Standard Methods for the Examination of Water and Wastewater 18th edn (Suppl.). Washington, D.C.: American Public Health Association. APHA (1992b) Standard Methods for the Examination of Water and Wastewater 18th edn. Washington, D.C.: American Public Health Association. Araki, S., Martin-Gomez, S., De Becares, E., Louis-Calaburg, E. and Rojo-Vazquez, F. (2001) Effect of high rate algal ponds on viability of Cryptosporidium parvum oocysts. Applied and Environmental Microbiology 67, 33223324. Dewedar, A. and Bahgat, M. (1995) Fate of faecal coliform bacteria in a wastewater retention reservoir containing Lemna gibba L. Water Research 29, 25982600. EPA (1983) Design Manual Municipal Wastewater Stabilization Ponds EPA 625/1-83-015. Cincinnati, OH: Environmental Protection Agency, Center for Environmental Research Information. Gersberg, M.R., Lyon, S.R., Brenner, R. and Elkins . B.V. (1987) Fate of viruses in articial wetlands. Applied and Environmental Microbiology 53, 731736. Grimalson, A.M., Smith, H.V., Thitai, W.N., Smith, P.G., Jackson, M.H. and Girdwood, R.W.A. (1993) Occurrence and removal of Cryptosporidium spp. oocysts and Giardia spp. cysts in Kenyan waste stabilization ponds. Water Science and Technology 27, 97104. Hammer, D.A. (1989) Constructed Wetlands for Wastewater Treatment: Municipal, Industrial and Agricultural. Chelsea, MI: Lewis Publishers, Inc. Kadlec, R.H. and Knight, R.L. (1996) Treatment Wetlands. Boca Raton, Florida, USA: CRC Press. Reed, S.C., Crites, R.W. and Midlebrooks, E.J. (1995) Natural Systems for Waste Management and Treatment 2nd edn. New York: McGrawHill, Inc. Rose, B.J., Gerba, C.P. and Jakubowski, W. (1991) Survey of potable water supplies for Cryptosporidium and Giardia. Environmental Science and Technology 25, 13931400.
2002 The Society for Applied Microbiology, Letters in Applied Microbiology, 34, 384387
Potrebbero piacerti anche
- Assessment of The Efficiency of Duckweed (Lemna Gibba) in Wastewater TreatmentDocumento7 pagineAssessment of The Efficiency of Duckweed (Lemna Gibba) in Wastewater TreatmentSandip AjmireNessuna valutazione finora
- Sublethal Stress in Escherichia Coli A Function of SalinitytDocumento6 pagineSublethal Stress in Escherichia Coli A Function of SalinitytgiuseppegnrNessuna valutazione finora
- Aquatic Phytoremediation of CCA and Copper Contaminated Water - Keith C. Et Al. 2007Documento6 pagineAquatic Phytoremediation of CCA and Copper Contaminated Water - Keith C. Et Al. 2007Javier Sulivan Paredes MurNessuna valutazione finora
- Application of Real-Time Quantitative PCR for the Detection of SelectedDocumento9 pagineApplication of Real-Time Quantitative PCR for the Detection of SelectedMiriam LeiNessuna valutazione finora
- Protistology: The Microfauna Communities and Operational Monitoring of An Activated Sludge Plant in ChinaDocumento5 pagineProtistology: The Microfauna Communities and Operational Monitoring of An Activated Sludge Plant in ChinaMurylu Dias dos SantosNessuna valutazione finora
- Degradasi PestisidaDocumento9 pagineDegradasi PestisidaFA InesNessuna valutazione finora
- Phylogenetically Diverse Acetaldehyde-Degrading Bacterial Community Deep Sea Water West Pacific OceanDocumento11 paginePhylogenetically Diverse Acetaldehyde-Degrading Bacterial Community Deep Sea Water West Pacific OceanAndrea EscobarNessuna valutazione finora
- Microbial Community Analysis of Four Swine Wastewater Anaerobic Lagoons Using Next-Generation DNA SequencingDocumento8 pagineMicrobial Community Analysis of Four Swine Wastewater Anaerobic Lagoons Using Next-Generation DNA SequencingDoulalas GiorgosNessuna valutazione finora
- 2008 - Detection of Salmonellae in Captive and Free-Ranging Turtles Using Enrichment Culture and Polymerase Chain Reaction - James P. GaertnerDocumento10 pagine2008 - Detection of Salmonellae in Captive and Free-Ranging Turtles Using Enrichment Culture and Polymerase Chain Reaction - James P. GaertnerNicolás SalaríNessuna valutazione finora
- Physiological Studies of Subtropical Mangrove ThraustochytridsDocumento8 paginePhysiological Studies of Subtropical Mangrove ThraustochytridsIsnaini PrihatiningsihNessuna valutazione finora
- Original Article Effect of Chlorine and Temperature On Larvicidal Activity of Cuban BacillusDocumento11 pagineOriginal Article Effect of Chlorine and Temperature On Larvicidal Activity of Cuban Bacillusmurillolillo2Nessuna valutazione finora
- Comparision of in Vivo and Invitro Method For MicrocyctinDocumento9 pagineComparision of in Vivo and Invitro Method For MicrocyctinHARDIK DESAINessuna valutazione finora
- Abundance of T4 Bacteriophages in Wastewater & SewageDocumento4 pagineAbundance of T4 Bacteriophages in Wastewater & SewagemarychuiyNessuna valutazione finora
- Siedel 1984 Aquacultural-EngineeringDocumento14 pagineSiedel 1984 Aquacultural-EngineeringJorge RodriguezNessuna valutazione finora
- The Use of Elements As A Substitute For Biomass in Toxicokinetic Studies in Small OrganismsDocumento7 pagineThe Use of Elements As A Substitute For Biomass in Toxicokinetic Studies in Small OrganismsBruno GomesNessuna valutazione finora
- Isolation Actinomycetes ANDRIANODocumento7 pagineIsolation Actinomycetes ANDRIANOBerenice AndrianoNessuna valutazione finora
- EJBO Volume 55 Issue 1 Pages 127-147Documento21 pagineEJBO Volume 55 Issue 1 Pages 127-147Royal GreenNessuna valutazione finora
- Waterborne Parasites: A Current Status From The Philippines: Research Open AccessDocumento8 pagineWaterborne Parasites: A Current Status From The Philippines: Research Open AccessTony DawaNessuna valutazione finora
- Ijaret 06 10 010Documento11 pagineIjaret 06 10 010IAEME PublicationNessuna valutazione finora
- s10327-023-01163-zDocumento4 pagines10327-023-01163-zbhanush.cimapNessuna valutazione finora
- Phytoremediation of Industrial Wastewater by Culturing Aquatic Macrophytes, Trapa Natans L. and Salvinia Cucullata RoxbDocumento9 paginePhytoremediation of Industrial Wastewater by Culturing Aquatic Macrophytes, Trapa Natans L. and Salvinia Cucullata RoxbArada SanNessuna valutazione finora
- Sampaio Et Al 2007Documento9 pagineSampaio Et Al 2007Bruna FacundesNessuna valutazione finora
- 2002-Flowcytometri Environmental SampleDocumento6 pagine2002-Flowcytometri Environmental SamplewiwienNessuna valutazione finora
- Effects of Fish Size and Biofiltration Techniques On Water Quality and Nitrogen Removal Efficiency in Recirculating Aquaculture SystemsDocumento11 pagineEffects of Fish Size and Biofiltration Techniques On Water Quality and Nitrogen Removal Efficiency in Recirculating Aquaculture Systemsrahma apriantyNessuna valutazione finora
- Characterization of E. Coli Strains Obtained From Wastewater EffluentDocumento8 pagineCharacterization of E. Coli Strains Obtained From Wastewater EffluentSEP-PublisherNessuna valutazione finora
- tmp1B25 TMPDocumento12 paginetmp1B25 TMPFrontiersNessuna valutazione finora
- tmp5EE1 TMPDocumento6 paginetmp5EE1 TMPFrontiersNessuna valutazione finora
- Mercury Uptake and Accumulation by Four Species of Aquatic PlantsDocumento4 pagineMercury Uptake and Accumulation by Four Species of Aquatic PlantsFebriani AabidahNessuna valutazione finora
- Contaminanti Hirdrofobi Toxicitate Alge PDFDocumento10 pagineContaminanti Hirdrofobi Toxicitate Alge PDFHoria MadearNessuna valutazione finora
- Test UploadDocumento8 pagineTest UploadAbi Diciembre BrionesNessuna valutazione finora
- Mirex Incorporation in The Environment: Ufi'Ake in Aquatic Organisms and Effects On The Rates of Photosynthesis and RespirationDocumento10 pagineMirex Incorporation in The Environment: Ufi'Ake in Aquatic Organisms and Effects On The Rates of Photosynthesis and RespirationSh1vaNessuna valutazione finora
- MATERIALS AND METHODS. Not EditeddocxDocumento6 pagineMATERIALS AND METHODS. Not EditeddocxRaquel Paleyan CalawenNessuna valutazione finora
- Polaromonas Naphthalenivorans Sp. Nov., A Naphthalene-Degrading Bacterium From Naphthalene-Contaminated SedimentDocumento5 paginePolaromonas Naphthalenivorans Sp. Nov., A Naphthalene-Degrading Bacterium From Naphthalene-Contaminated SedimentAshok Singh MauryaNessuna valutazione finora
- 17 Full PDFDocumento9 pagine17 Full PDFPriscilia YuniarNessuna valutazione finora
- Assignment of Lemna Gibba L. (Duckweed) Bioassay For in Situ Ecotoxicity AssessmentDocumento15 pagineAssignment of Lemna Gibba L. (Duckweed) Bioassay For in Situ Ecotoxicity AssessmentJEANPIERRE ALEJANDRO CHIRITO MAGUIÑANessuna valutazione finora
- Mixed SedimentDocumento15 pagineMixed SedimentprashmceNessuna valutazione finora
- Influence of Treatments in The Quality of Nile Tilapia (Oreochromis Niloticus) FilletsDocumento8 pagineInfluence of Treatments in The Quality of Nile Tilapia (Oreochromis Niloticus) Filletsmarine2006Nessuna valutazione finora
- Lyngby ADocumento12 pagineLyngby ANatalia DonțuNessuna valutazione finora
- Algae/Bacteria Ratio in High-Rate Ponds Used For Waste TreatmentDocumento8 pagineAlgae/Bacteria Ratio in High-Rate Ponds Used For Waste Treatment'Hady' HadiyantoNessuna valutazione finora
- Appl. Environ. Microbiol.-2000-Antón-3052-7 PDFDocumento6 pagineAppl. Environ. Microbiol.-2000-Antón-3052-7 PDFEliana Gómez RománNessuna valutazione finora
- Artigo 4 Cardinali-Rezende Suino 2012 PDFDocumento9 pagineArtigo 4 Cardinali-Rezende Suino 2012 PDFJuliana Cardinali RezendeNessuna valutazione finora
- Use of Waste Water For Aquaculture - An Experimental Field Study at A Sewage-Treatment PlantDocumento0 pagineUse of Waste Water For Aquaculture - An Experimental Field Study at A Sewage-Treatment PlantjfejfeNessuna valutazione finora
- Genetic Diversity of Total, Active and Culturable Marine Bacteria in Coastal SeawaterDocumento11 pagineGenetic Diversity of Total, Active and Culturable Marine Bacteria in Coastal SeawaterMelody ChristineNessuna valutazione finora
- Cyclospora Cayetanensis Travels in Tap Water On Italian TrainsDocumento7 pagineCyclospora Cayetanensis Travels in Tap Water On Italian TrainsIsabella GonçalvesNessuna valutazione finora
- Calderon Et Al 2013Documento6 pagineCalderon Et Al 2013Charles OlayaNessuna valutazione finora
- Alambra Et Al - Review & Comments Edited 01-22 EditDocumento8 pagineAlambra Et Al - Review & Comments Edited 01-22 EditKenneth_Alambr_1485Nessuna valutazione finora
- Appl. Environ. Microbiol. 2008 Harmer 3895 8Documento5 pagineAppl. Environ. Microbiol. 2008 Harmer 3895 8DbaltNessuna valutazione finora
- Brogan & PartnersDocumento7 pagineBrogan & Partnerssabzar_cordNessuna valutazione finora
- Enumeration and Identification of Standard Plate Count Bacteria in Raw Water SuppliesDocumento7 pagineEnumeration and Identification of Standard Plate Count Bacteria in Raw Water SuppliesLiza Lolita PutriNessuna valutazione finora
- Leonard 2000Documento12 pagineLeonard 2000juanr25Nessuna valutazione finora
- Characterization of Pseudomonas Spp. From Seawater of The Southwest Coast of TurkeyDocumento9 pagineCharacterization of Pseudomonas Spp. From Seawater of The Southwest Coast of TurkeyDrashua AshuaNessuna valutazione finora
- Plankton survey assesses Calabar River for recreationDocumento8 paginePlankton survey assesses Calabar River for recreationfrfunkNessuna valutazione finora
- Colletotrichum Gloeosporioides From Mango Ataulfo: Morphological, Physiological, Genetic and Pathogenic AspectsDocumento7 pagineColletotrichum Gloeosporioides From Mango Ataulfo: Morphological, Physiological, Genetic and Pathogenic AspectsresearchinbiologyNessuna valutazione finora
- CR (VI) Reduction in A Chromate-Resistant Strain of Candida Maltosa Isolated From The Leather IndustryDocumento6 pagineCR (VI) Reduction in A Chromate-Resistant Strain of Candida Maltosa Isolated From The Leather IndustryEdilberto Murrieta LunaNessuna valutazione finora
- Evaluation of Genetic Damage Induced by Glyphosate Isopropylamine Salt Using Tradescantia Bioassays - Alvarez Et Al. - 2011Documento4 pagineEvaluation of Genetic Damage Induced by Glyphosate Isopropylamine Salt Using Tradescantia Bioassays - Alvarez Et Al. - 2011vmsolartecNessuna valutazione finora
- Antimicrobial Activity of Scorpaenopsis venosaDocumento6 pagineAntimicrobial Activity of Scorpaenopsis venosaizzaaaaawNessuna valutazione finora
- Im1104 0267Documento8 pagineIm1104 0267Crista María CanoNessuna valutazione finora
- Monoraphid and Naviculoid Diatoms from the Coastal Laurentian Great LakesDa EverandMonoraphid and Naviculoid Diatoms from the Coastal Laurentian Great LakesAndrzej WitkowskiNessuna valutazione finora
- Periphyton: Functions and Application in Environmental RemediationDa EverandPeriphyton: Functions and Application in Environmental RemediationNessuna valutazione finora
- 10 1 1 26 2529Documento13 pagine10 1 1 26 2529Sandip AjmireNessuna valutazione finora
- Acids, Bases, & SaltsDocumento30 pagineAcids, Bases, & SaltsSandip AjmireNessuna valutazione finora
- Time Table Summer 2014Documento1 paginaTime Table Summer 2014sandipajmireNessuna valutazione finora
- Hum Jante Hai Aap Hai BekaraarDocumento3 pagineHum Jante Hai Aap Hai BekaraarSandip AjmireNessuna valutazione finora
- Irrigation Engineering: Prof - Sandip Ajmire M. TechDocumento24 pagineIrrigation Engineering: Prof - Sandip Ajmire M. TechSandip AjmireNessuna valutazione finora
- "Is Globalization Civilizing, Destructive, or Feeble?" - Mauro GuillénDocumento24 pagine"Is Globalization Civilizing, Destructive, or Feeble?" - Mauro GuillénSandip AjmireNessuna valutazione finora
- The Nervous System and Reflex ArcDocumento35 pagineThe Nervous System and Reflex ArcSandip AjmireNessuna valutazione finora
- Magnetic Effects of Electric CurrentDocumento17 pagineMagnetic Effects of Electric CurrentKunalKaushik100% (2)
- Intro Animal Physiology Ch-2.1Documento97 pagineIntro Animal Physiology Ch-2.1Jojo MendozaNessuna valutazione finora
- Contacts Third YearDocumento6 pagineContacts Third YearSandip AjmireNessuna valutazione finora
- Light PowerpointDocumento34 pagineLight PowerpointSalmizam Izam100% (1)
- Irrigation Eng11Documento28 pagineIrrigation Eng11Sandip AjmireNessuna valutazione finora
- VISUAL CRYPTOGRAPHY: SECRET SHARING WITHOUT A COMPUTERDocumento22 pagineVISUAL CRYPTOGRAPHY: SECRET SHARING WITHOUT A COMPUTERsanthanm143Nessuna valutazione finora
- Cells and Life ProcessesDocumento5 pagineCells and Life ProcessesSandip AjmireNessuna valutazione finora
- Chemical EquationsDocumento78 pagineChemical EquationsSanchay SaxenaNessuna valutazione finora
- Cells and Life ProcessesDocumento5 pagineCells and Life ProcessesSandip AjmireNessuna valutazione finora
- Semi Final 4Documento90 pagineSemi Final 4Sandip AjmireNessuna valutazione finora
- Acids, Bases, & SaltsDocumento30 pagineAcids, Bases, & SaltsSandip AjmireNessuna valutazione finora
- Phytoremediation FinalDocumento28 paginePhytoremediation FinalSandip AjmireNessuna valutazione finora
- Ncet Ganesh 2012Documento44 pagineNcet Ganesh 2012Sandip AjmireNessuna valutazione finora
- Irrigation Engineering: Prof - Sandip Ajmire M. TechDocumento24 pagineIrrigation Engineering: Prof - Sandip Ajmire M. TechSandip AjmireNessuna valutazione finora
- Irrigation Engineering: Prof - Sandip Ajmire M. TechDocumento24 pagineIrrigation Engineering: Prof - Sandip Ajmire M. TechSandip AjmireNessuna valutazione finora
- Semi Final 9-10-2012Documento62 pagineSemi Final 9-10-2012Sandip AjmireNessuna valutazione finora
- Crypto PPT 3semi 4Documento30 pagineCrypto PPT 3semi 4Sandip AjmireNessuna valutazione finora
- PP T 0000018Documento27 paginePP T 0000018Sandip AjmireNessuna valutazione finora
- WaterDocumento10 pagineWaterSandip AjmireNessuna valutazione finora
- Digital Water Mark 2-9Documento19 pagineDigital Water Mark 2-9Sandip AjmireNessuna valutazione finora
- Studies On Treatment of Waste Water by Phytoremedation ProcessDocumento56 pagineStudies On Treatment of Waste Water by Phytoremedation ProcessSandip AjmireNessuna valutazione finora
- Semi Final 4Documento52 pagineSemi Final 4Sandip AjmireNessuna valutazione finora