Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Assignment of Blood Flow
Caricato da
arsalanbookDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Assignment of Blood Flow
Caricato da
arsalanbookCopyright:
Formati disponibili
Fluid Mechanics Of Blood Flow
Blood, which exhibits a highly complex, flow-dependent physical, and chemical constitution, is composed of plasma, a Newtonian liquid of viscosity 1mPa s, RBCs, White Blood Cells (WBCs) and platelets. Approximately 90% of the plasma is water, and the rest is composed of dissolved substances, primarily proteins, e.g., albumin, globulin and fibrinogen. The volume percentage of plasma typically accounts for 55% and the remaining 45% is almost entirely composed of RBCs. Only up to 1% of the total cell mass in blood is formed by platelets and WBCs. Platelets are 24 m disk-shaped particles that are involved in the cellular mechanisms that initiate the wound healing cascade through the formation of blood clots. The WBCs are responsible for the bodys defense against infectious disease and foreign materials as part of the immune system. RBCs contain hemoglobin which has the function of distributing oxygen to the tissues and organs. Non-physiological conditions, in particular high shear stresses over a prolonged time, can cause damage to the RBCs. Characteristic shear stresses acting on blood in the human vessels as opposed to artificial organs. These stresses lie within a range of 0.11 Pa in healthy human veins and 120 Pa in healthy human arteries, but this value can increase up to 50 Pa in narrowed arteries caused by stenotic lesions. In artificial blood pumps, typical values are found in a range of 11,000 Pa
The Mechanics of Blood Circulation
Mechanics is the study of motion (or equilibrium) and the forces that causes it. The blood moves in the blood vessels, while the heart serves as the pump for the blood. The vessel walls of the heart are elastic and are movable, therefore causing the blood and the wall to exert forces on each other which in turn influence their respective motion. Therefore to understand the mechanics of circulation of the heart, it will be worth the while to go through a review of basic mechanics of fluid, and elastic solids (momentum) and the nature of the forces exerted between two moving substances in contact. Another quantity of interest in describing the motion of a particle is its velocity. The velocity basically is the rate of change of the position of an object with time.
Blood velocities in arteries are higher during systole than during diastole. One parameter to quantify this difference is pulsatility index(PI), which is equal to the difference between the peak systolic velocity and the minimum diastolic velocity divided by the mean velocity during the cardiac cycle.
From a historical perspective, work on the systemic circulation has gone through a number of distinct eras: from the ancient era where blood was thought to be manufactured in the heart and then dispatched to the rest of the body, to the next era where it was recognized that blood is actually in continuous circulation through the body, then the era of steady Poiseuille flow where the focus was on the details of flow within the blood vessels, to the era of pulsatile flow, first in a rigid tube, then in an elastic tube, which led to the era of wave propagation and wave reflections and, finally, to the modern era where computer power coupled with the Navier-Stokes equations has made it possible to simulate blood flow in every possible configuration. It is interesting that every era was marked by an unwavering commitment to the subject of the day, all else being regarded as secondary, just as the pulsatile nature of blood flow was regarded as secondary in the era of Poiseuille flow, or as wave reflections were regarded as secondary in the early days of pulsatile flow. Secondary issues were actually seen as aberrations in the design of the cardiovascular system, even with some negative connotation. Indeed, to this day we continue to think of wave reflections in this negative way, as an added cost to the system, or an afterthought in the design of the system. Wave reflections have yet to be embraced in our minds as an intrinsic part of the system, not secondary or negative in any way, but simply an integral part of the system, essential as any other in the efficient working of the system, and as such they may actually be used by the system to some advantage. In the modern era our commitment is to the Navier-Stokes equations and to our computer power to solve them under every conceivable condition, thereby making it possible to simulate most of the blood flow phenomena which we observe within the human body. As in previous eras we feel that we have the subject essentially in hand, though, again, with the exception perhaps of some secondary issues. In what follows we consider one such issue, namely that of control and regulation of fluid flow within the human body, more specifically the control and regulation of blood flow. While blood flow is certainly governed by the Navier-Stokes equations, it is at no time governed by the Navier-Stokes equations alone. Blood flow is part of a closed-loop control system, or more precisely it is under a hierarchy of local and global control loops in which the primary object is control of pressure and flow rate. While the Navier-Stokes equations govern the relation between pressure and flow, it is an open-loop relation which they govern. In that relation, a change in pressure produces a change in flow and there the matter ends. There is no provision for any feedback from the change in flow back to the change in pressure, yet in the physiological system feedback loops are ever present. Feedback loops in the cardiovascular system are mediated by a number of neural and humoral control mechanisms. As in a man-made control system, each of these loops is associated with a controlled variable, a sensor, and a controller that is able to affect the value of the controlled
variable. While a great deal has been learned about certain elements of these control systems, much is still unknown. Typically, a controlled variable such as blood pressure or heart rate is identified and some of the sensors associated with it are located, but the control system loop governing that variable can rarely be sketched out or isolated in complete form. This is not only because some of the sensors or pathways are unknown but because in most if not all cases the loops involved, rather than being distinct closed loops, may typically be interlaced with each other. The control systems for heart rate and stroke volume, for example, may have both local and central loops and these loops may interlace with each other as well as with others associated perhaps with the control of blood pressure and pCO2. To deal with blood flow in its full breadth, therefore, it is necessary to integrate hemodynamics with the full dynamics of the system because the integrity of blood flow in any organ or system depends not only on hemodynamics within the blood vessels of that organ or system but also on the full dynamics of the system as a whole. The first of these is governed by the Navier-Stokes equations, the second by the control loops and mechanisms associated with blood flow in that particular organ. Put another way, while satisfying the Navier-Stokes equations is a necessary condition for an accurate description of blood flow within the vascular bed of an organ, it is not a sufficient condition for a description of the overall dynamics of that flow in the context of control and regulation, or in the context of what is the ultimate objective of blood flow in that organ, namely cell perfusion. Arrhythmia, in all its forms, is a familiar example of this dichotomy. It affects the harmony between the pumping rhythm of the heart and the compliance of the aorta, thereby affecting cardiac output and the dynamics of the systemic circulation. In the heart, similarly, a disruption in the harmony between the pulsatile rhythm of the input pressure driving the flow into the coronary circulation, the pulsatile rhythm of the contracting cardiac muscle and its effect on coronary vessels imbedded within it, and the combination of wave propagation and wave reflections within the coronary vascular tree, will lead to a failure of the dynamics of the coronary circulation while the flow within individual vessels is continuing to satisfy the NavierStokes equations. A much simplified version of this scenario where the dynamics of the flow in a vascular tree is disturbed by vasodilatation or vasoconstriction at the peripheral levels of the tree. The result is a change in the distribution of admittance along the tree and therefore a change in the dynamics of blood flow through the tree. The flow in individual vessel segments remains orderly in the sense that it continues to satisfy the Navier-Stokes equations. Thus, while the overall dynamics of the flow have been affected, hemodynamics within the tree have not. Admittance distribution in an 11-level tree model (top) and how it is affected by vasodilatation or vasoconstriction in the peripheral vessels (bottom).
In sudden cardiac death there is a precipitous fall in cardiac output to levels that can no longer sustain cerebral or cardiac function. By definition, the timing of this failure cannot be attributed to any prevailing coronary artery disease, so the disease if present is seen only as a participant in but not the immediate cause of the event. According to currently held view, coronary artery disease is seen as a precursor to conditions of myocardial ischemia and infarction, leading to a disruption in heart rhythm, fall in cardiac output, fall in coronary blood supply and finally heart failure. A difficulty with this course of events is that it proposes that a fall in cardiac output is followed by a fall in coronary blood flow. The mechanism for this sequence is not clear if indeed possible, because if the fall in cardiac output leads to a drop in aortic pressure, it is well known that autoregulation of coronary blood flow ensures that this does not lead immediately to a fall in coronary blood flow. Because of autoregulation, a fall in cardiac output is more likely to be the consequence rather than the cause of a fall in coronary blood flow, the latter being precipitated by a disruption in the dynamics of the coronary circulation produced by coronary artery disease, cardiac damage, or a disruption in neural activity.
Current view of the course of events leading to sudden cardiac death in which a fall in cardiac output is followed by a fall in coronary blood supply. A more likely course of events leading to sudden cardiac death in which the fall in cardiac output is the consequence rather than the cause of a fall in coronary blood supply, and the primary trigger is a disruption in the dynamics of the coronary circulation. Thus, the integrity of blood flow in its widest sense depends in the first place on hemodynamics within the vascular bed but ultimately on the dynamics of the system as a whole, and in the same way that the first of these may be disrupted by a vascular pathology, the second may be disrupted by what may be termed a dynamic pathology. Arrhythmia is a clear example of a dynamic pathology while sudden cardiac death is, ultimately, the result of a dynamic pathology. A dynamic pathology is not unlike a structural or functional pathology in a tissue or organ, but it deals specifically with dynamics. Furthermore, it deals with the dynamics of an integrated system as a whole, such as the dynamics of the systemic or of the coronary circulation, including all the feedback loops involved in the control and regulation of the system in each case. Thus, an atherosclerotic lesion in a coronary artery is a vascular pathology, but the ultimate significance of this lesion lies only in the dynamic pathology which it produces, more specifically in the way the lesion affects the dynamics of the coronary circulation as a whole, and in particular in the way it affects the ability of the coronary circulation to provide the heart with sufficient blood supply. If the occlusive progression of the lesion is sufficiently slow so that the occlusion is
compensated for by vascular restructuring, for example, the dynamic pathology may be minimal or nonexistent. Thus, the presence of vascular pathology does not necessarily imply the presence of dynamic pathology, but the reverse is not necessarily true, namely the absence of vascular pathology does not necessarily imply the absence of dynamic pathology. Perhaps the most celebrated example of a dynamic pathology is the phenomenon of a broken heart which has recently made the transition from the realm of folklore to that of medicine where it is now accepted as a legitimate clinical syndrome. Following a multi-patient clinical study, Wittstein et al [94] reported that Emotional stress can precipitate severe, reversible left ventricular dysfunction in patients without coronary disease. Exaggerated sympathetic stimulation is probably central to the cause of this syndrome. Because of the absence of coronary artery disease in these patient, the precipitating pathology in this case is again a dynamic pathology as in the case of sudden cardiac death, namely a disruption in the dynamics of the coronary circulation followed by a fall in coronary blood supply and a fall in cardiac output. One of the most important aspects of dynamic pathologies is that unlike structural pathologies they do not leave a footprint after they have resolved or produced their damage. Following sudden cardiac death, indeed following any death attributed to heart disease, only structural pathologies can usually be found and they become the focus of attention. Any dynamic pathologies involved are not in evidence because they are no longer at play. This is the reason for which the broken heart syndrome has taken so long to be accepted by the medical community. The concept of a dynamic pathology, which is a failure in the overall dynamics of a dynamic system, is well familiar in science and engineering but is rather foreign in the clinical setting where the focus is on the more tangible hemodynamics. The broken heart syndrome, with the distinction of being transient and reversible, offers the opportunity to make the dynamic pathology more tangible, that is it makes it possible to detect the dynamic pathology while it is at play and in pure form, because the syndrome is not associated with coronary artery disease. Bernoulli's Principle and Energetics of Flowing Blood Because flowing blood has mass and velocity it has kinetic energy (KE). This KE is proportionate to the mean velocity squared (V2; from KE = mV2). Furthermore, as the blood flows inside a vessel, pressure is exerted laterally against the walls of the vessel; this pressure represents the potential or pressure energy (PE). The total energy (E) of the blood flowing within the vessel, therefore, is the sum of the kinetic and potential energies (assuming no gravitational effects) as shown below. E = KE + PE (where KE V2) Therefore, E V2 + PE
There are two important concepts that follow from this relationship. 1. Blood flow is driven by the difference in total energy between two points. Although pressure is normally considered as the driving force for blood flow, in reality it is the total energy that drives flow between two points (e.g., longitudinally along a blood vessel or across a heart valve). Throughout most of the cardiovascular system, KE is relatively low, so for practical purposes, it is stated that the pressure energy (PE) difference drives flow. When KE is high, however, adding KE to the PE significantly increases the total energy, E. To illustrate this, consider the flow across the aortic valve during cardiac ejection. Late during ejection, the intraventricular pressure (PE) falls slightly below the aortic pressure (PE), nevertheless, flow continues to be ejected into the aorta. The reason for this is that the KE of the blood as it moves across the valve at a very high velocity ensures that the total energy (E) in the blood crossing the valve is higher than the total energy of the blood more distal in the aorta.
2. Kinetic energy and pressure energy can be interconverted so that total energy remains unchanged. This is the basis of Bernoulli's Principle. This principle can be illustrated by a blood vessel that is suddenly narrowed then returned to its normal diameter. In the narrowed region (stenosis), the velocity increases as the diameter decreases. Quantitatively, V 1/D2because flow (F) is the product of mean velocity (V) and vessel cross-sectional area (A) (F = V A), and A is directly related to diameter (D) (or radius, r) squared (from A = r2). If the diameter is reduced by one-half in the region of the stenosis, the velocity increases 4-fold. Because KE V2, the KE increases 16-fold. Assuming that the total energy is conserved within the stenosis (E actually decreases because of resistance), then the 16-fold increase in KE must result in a proportionate decrease in PE. Once past the narrowed segment, KE will revert back to its pre-stenosis value because the post-stenosis diameter is the same as the pre-stenosis diameter and flow is conserved. Because of the resistance of the stenosis, and the likelihood of turbulence, the post-stenosis PE and E will both fall. To summarize this concept, blood flowing at higher velocities has a higher ratio of kinetic energy to potential (pressure) energy.
An interesting, yet practical application of Bernoulli's Principle is found when blood pressure measurements are made from within the ascending aorta. As described above, during ventricular ejection, the velocity and hence kinetic energy of the flowing blood is very high.
The instantaneous blood pressure that is measured within the aorta will be very different depending upon how the pressure is measured. As illustrated to the right, if a catheter has an endport (E) sensor that is facing the flowing stream of blood, it will measure a pressure that is significantly higher than the pressure measured by a side-port (S) sensor on the same catheter. The reason for the discrepancy is that the end-port measures the total energy of the flowing blood. As the flow stream "hits" the end of the catheter, the kinetic energy (which is high) is converted to potential (or pressure) energy, and added to the potential energy to equal the total energy. The side-port will not be "hit" by the flowing stream so kinetic energy is not converted to potential energy. The side-port sensor, therefore, only measures the potential energy, which is the lateral pressure acting on the walls of the aorta. The difference between the two types of pressure measurements can range from a few mmHg to more than 20 mmHg depending upon the peak velocity of the flowing blood within the aorta.
BLOOD FLOW AT LOW REYNOLDS NUMBERS
In capillary blood vessels, the diameter of the undeformed cell is about equal to or greater than the capillary diameter, which varies between 4 and 10 micro meter. The cells usually travel in single file and may fill most of the lumen, leaving only a thin layer of plasma between the cell and the vessel wall. The red blood cells are quite deformed from their resting biconcave disk shape, and the extent of the deformation is dependent on the appliedpressure gradient.. The redblood-cell membrane is a viscoelastic material. Under steadyflow conditions, the elastic properties of the membrane dominate over its viscous properties. In the situations discussed herein, only the elastic properties of the red-cell membrane are taken into consideration. We
assume that the red blood cell'has a constant surface area and volume, that the flow properties and 'the shape of the red cell are quasi-steady, and that the fluid within the cell produces only a constant-pressure field. The densities of the red cells and plasma are nearly equal, so capillary flow is regarded as a suspension of neutrally buoyant particles. Since the typical Reynolds number in the capillaries is about 0.01 or less, only low-Reynolds-number flows need be considered in capillary-flow models . Depending on the thickness of the gap between the cells and the vessel wall, either the Stokes equations are used in full or Reynolds lubrication theory is applied to describe the plasma flow.
BLOOD FLOW AT HIGH REYNOLDS NUMBERS
Blood flow in human circulation is normally not turbulent. The term "high-Reynolds-number flow" is used here to indicate transient flows in the large blood vessels in which the effects of inertia are significant. The wave propagation in the major blood vessels and the intermittent flow in the heart are unsteady flows in which inertia plays a definite role. Although blood flow is normally not a fully developed turbulence, secondary flows at branches and bends occur in a transient and irregular manner. In the arterial system the mean pressure is normally sufficient so that the arteries remain distended during the entire heart cycle. A wave of pressure and flow emanates from the heart at each peak and pulses toward the peripheral circulation. This wave propagation has received a great deal of attention over the years, starting with an early paper by Euler written in 1 775 and published posthumously in 1 862 (Euler 1 862). In the venous system the mean pressure is lower, and the veins are subject to collapse under certain conditions. A very interesting chapter of theory and experiments has developed over the last two decades in the study of flow in collapsible tubes. Detailed or local flow patterns in the circulatory system are of interest in two principal situations. First, flows through natural and artificial heart valves are of interest from the standpoint of diagnosis and design of valve replacements. Details of flow patterns may affect the trauma of the blood and eventually the vessel walls. Second, much attention has been given to the flow in branches and at bends in relation to atherosclerosis. It is clear that the locations of initial development of atherosclerosis are regularly associated with the inner curvatl:lre of bends and in regions with a tendency toward flow separation at bifurcations of blood vessels. The mechanism by which the fluid-mechanica] flow patterns influence the development of atherosclerosis is still not entirely clear, but current research suggests that it may be associated with the effects of transient shear stresses on the endothelial cell layer. Reynolds numbers for some real-life examples Blood flow in brain ~ 100 Blood flow in aorta ~ 1000
LAMINAR FLOW OF BLOOD
Laminar flow is the normal condition for blood flow throughout most of the circulatory system. It is characterized by concentric layers of blood moving in parallel down the length of a blood vessel. The highest velocity (Vmax) is found in the center of the vessel. The lowest velocity (V=0) is found along the vessel wall. The flow profile is parabolic once laminar flow is fully developed. This occurs in long, straight blood vessels, under steady flow conditions.
One practical implication of parabolic, laminar flow is that when flow velocity is measured using a Doppler flowmeter, the velocity value represents the average velocity of a cross-section of the vessel, not the maximal velocity found in the center of the flow stream. The orderly movement of adjacent layers of blood flow through a vessel helps to reduce energy losses in the flowing blood by minimizing viscous interactions between the adjacent layers of blood and the wall of the blood vessel. Disruption of laminar flow leads to turbulence and increased energy losses. The movement of blood through the vessels. It is pulsatile in the large arteries, diminishing in amplitude as it approaches the capillaries. In the veins it is non-pulsatile. The flow in arteries is the result of ventricular ejection; in the veins it is a result of a number of factors including respiratory movement, muscle compression and the small residuum of arterial pressure. Recently it has been shown experimentally that transport of heat and gas (specifically oxygen and helium) are augmented in the laminar flow of aqueous suspensions of polystyrene spheres 50 and 150 micrometer in diameter. In this report, data on heat and gas transport are correlated. Application of this correlation to flowing blood leads to the following conclusions. There is no significant augmentation of oxygen and heat transport in flowing blood even at shear rates much higher than physiological shear rates; an observation which is in accord with the experimental results. The augmentation of the diffusion coefficient of plasma proteins in flowing blood, though not very high, appears to be measurable. Of the total measured augmentation of about 6000--30 000% in platelet diffusivity in flowing blood, quoted from the literature, about 500% is attributable from this correlation to fluid mechanical forces, and the balance is hypothetically attributed to other forces (electrical or biochemical) present in blood.
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Assignment of Resiration SyetemDocumento5 pagineAssignment of Resiration SyetemarsalanbookNessuna valutazione finora
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Ambulance Medical EquipmentDocumento32 pagineAmbulance Medical EquipmentarsalanbookNessuna valutazione finora
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- 1000 Most Common Words (SAT)Documento70 pagine1000 Most Common Words (SAT)grellian95% (20)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (894)
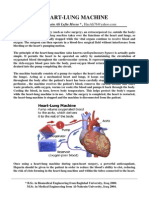
- Heart-Lung MachineDocumento4 pagineHeart-Lung Machinearsalanbook100% (2)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- European Society of Cardiology Congress - Augast.2022Documento36 pagineEuropean Society of Cardiology Congress - Augast.2022Giorgi PopiashviliNessuna valutazione finora
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- 2009 Handbook of Nurse Anesthesia, John J. Nagelhout, Karen Plaus PDFDocumento840 pagine2009 Handbook of Nurse Anesthesia, John J. Nagelhout, Karen Plaus PDFNoval Zain100% (1)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Clinical Indications, Treatment and Current PracticeDocumento14 pagineClinical Indications, Treatment and Current PracticefadmayulianiNessuna valutazione finora
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Unit1Documento40 pagineUnit1Lily KkNessuna valutazione finora
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- Nayeli Sierra Ga FinalDocumento7 pagineNayeli Sierra Ga Finalapi-300744043Nessuna valutazione finora
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- Beginning Medical Transcription (2nd Ed.) : Cardiopulmonary Reports Ent and Ophthalmology ReportsDocumento7 pagineBeginning Medical Transcription (2nd Ed.) : Cardiopulmonary Reports Ent and Ophthalmology ReportsRoderick RichardNessuna valutazione finora
- Diabetic DysipidemiaDocumento8 pagineDiabetic DysipidemiaAyur SuccessNessuna valutazione finora
- Heat Therapy To Reduce Chest Pain Among Patient With ACSDocumento10 pagineHeat Therapy To Reduce Chest Pain Among Patient With ACSRavitman RavitmanNessuna valutazione finora
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Top 10 Ways to Lower Your Triglycerides NaturallyDocumento2 pagineTop 10 Ways to Lower Your Triglycerides NaturallySumit DhingraNessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Mapeh 7-With TosDocumento5 pagineMapeh 7-With TosLorraine Calvez Donio100% (1)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- ExerciseDocumento7 pagineExerciseAjay JethwaNessuna valutazione finora
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Medicinal Flora in Holy QuranDocumento16 pagineMedicinal Flora in Holy QuranMuhammad Zakiy FadlullahNessuna valutazione finora
- A Study To Assess The Knowledge About Risk Factors and Warning Signs of Acute Coronary Syndrome Among Patients Admitted in Cardiac Medical Unit at Sctimst, Triv AndrumDocumento72 pagineA Study To Assess The Knowledge About Risk Factors and Warning Signs of Acute Coronary Syndrome Among Patients Admitted in Cardiac Medical Unit at Sctimst, Triv AndrumNise Mon KuriakoseNessuna valutazione finora
- 4e 2ndproof ch7 PDFDocumento19 pagine4e 2ndproof ch7 PDFJessa AquitanNessuna valutazione finora
- Medical-Surgical Management For RHDDocumento4 pagineMedical-Surgical Management For RHDJansen Arquilita RiveraNessuna valutazione finora
- Hypertension: Management and TreatmentDocumento9 pagineHypertension: Management and TreatmentSheila May Teope SantosNessuna valutazione finora
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Cardiovascular NotebookDocumento140 pagineCardiovascular NotebookChantal CarnesNessuna valutazione finora
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- Final Announcement ACIC ACEM SCU CME PDFDocumento23 pagineFinal Announcement ACIC ACEM SCU CME PDFFredik JackyNessuna valutazione finora
- ACC Handbook Ascvd Type 2 Diabetes: On andDocumento10 pagineACC Handbook Ascvd Type 2 Diabetes: On andZH. omg sarNessuna valutazione finora
- DLP-Bohol - Science9 Q1 W2 D4Documento6 pagineDLP-Bohol - Science9 Q1 W2 D4Marfe MontelibanoNessuna valutazione finora
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Completely Reverse Heart Disease With This Revolutionary TreatmentDocumento8 pagineCompletely Reverse Heart Disease With This Revolutionary TreatmentSamee's Music100% (3)
- Cardiac RehabitationDocumento4 pagineCardiac Rehabitationsundar_kumar0Nessuna valutazione finora
- 16 Vascular Diseases of Nervous System-QDocumento24 pagine16 Vascular Diseases of Nervous System-QAdi PomeranzNessuna valutazione finora
- Case Stydy Angina PectorisDocumento46 pagineCase Stydy Angina PectorissharenNessuna valutazione finora
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Glenn R Gibson - Food Science and Technology Bulletin - Functional Foods. Vol. 1 PDFDocumento107 pagineGlenn R Gibson - Food Science and Technology Bulletin - Functional Foods. Vol. 1 PDFguiovanaNessuna valutazione finora
- 2000 Bookmatter TextbookOfAngiologyDocumento19 pagine2000 Bookmatter TextbookOfAngiologydavidmarkovic032Nessuna valutazione finora
- Stress and Immune SystemDocumento15 pagineStress and Immune SystemRhishabhNessuna valutazione finora
- Health Benefits of Wheat and Its Nutritional ValueDocumento13 pagineHealth Benefits of Wheat and Its Nutritional ValueJaime BallesterosNessuna valutazione finora
- What Is Coronary Angiography?Documento4 pagineWhat Is Coronary Angiography?emman_abzNessuna valutazione finora
- Health Combinat 2Documento58 pagineHealth Combinat 2mariuscornelNessuna valutazione finora
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)