Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Bowen's Reaction Series
Caricato da
Denie PutraDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Bowen's Reaction Series
Caricato da
Denie PutraCopyright:
Formati disponibili
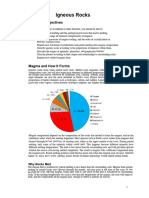
BOWENS REACTION SERIES Igneous rocks are made up of minerals that reflect the composition of the magma/lava from
which they crystallized out of, as well as the temperature of the magma/lava. Remember, the TEXTURE of an igneous rock is essentially a function of how rapidly (or slowly) the magma cooled, not what it is composed of. A magma that cools rapidly, i.e., one that reaches the surface of the Earth, is going to be inherently very fine grained. The individual mineral grains that make up this type of igneous rock are generally too fine to see with the eye. These are known as extrusive or volcanic rocks. A magma that cools slowly, i.e., one that never reaches the Earths surface, will generally have enough time to allow the individual mineral grains to grow to a size that is visible with the eye. The degree of coarseness, that is, how large any given mineral grain may grow, is generally a reflection of how slowly it cooled (slower cooling = bigger grains, quicker cooling = finer grains). So why do igneous rocks (volcanic or plutonic) exhibit different types of compositions? How does a single magma source produce chemically different types of igneous rocks? As a general rule, most magmas contain varying amounts of silica (SiO2) along with other elements, most commonly, Al, Fe, Ca, Na, K, and Mg. The silica and other elements combine to form various silicate minerals. The most common minerals making up igneous rocks are; Plagioclase feldspar Ca, Na, Al Silicate Orthoclase feldspar K,Al Silicate Olivine Fe, Mg Silicate Quartz Silicate Amphiboles (e.g. hornblende) Ca, Na, Mg, Fe, Al Silicate Pyroxenes (e.g. augite) Ca, Na, Mg, Fe, Al Silicate Biotite K, Mg, Fe, Al Silicate Muscovite K, Al Silicate [Notice that all of these minerals simply represent different combinations of the above-listed elements!] Laboratory experiments have confirmed that as a magma cools, minerals will crystallize out in a predictable sequence. This sequence is known as Bowens Reaction Series. It is made up of two trends, the Discontinuous Reaction Series and the Continuous Reaction Series (see attached diagram). Discontinuous Reaction Series This side of the diagram is composed of Olivine, Pyroxenes (you have not learned any of these), Amphiboles (hornblende is the most common), and Biotite mica. All of these minerals are frequently referred to as Ferromagnesian minerals, that is, they all contain Fe and Mg, and are therefore very dark colored. These minerals crystallize from the melt but subsequently react with the remaining melt(liquid) as the temperature changes. For example, olivine has the highest crystallization/melting point, hence is first to crystallize out into solid form as a magma cools. But as the remaining liquid portion of the melt continues to cool, the olivine crystals become unstable and react with the melt to form a new mineral group (the pyroxenes). The crystalline olivine may be partially to completely destroyed (used up) in reacting with the remaining melt(liquid) to produce a new more stable mineral. In this case, pyroxene crystallizes out at a slightly lower melting/crystallization point. And this reaction trend may continue as the temperature continues to drop, following the sequence on the diagram [olivine pyroxene amphibole biotite].
Continuous Reaction Series This side of the diagram is composed entirely of the Plagioclase Feldspars. Although you have only learned it as a single species, it is actually divided up into several names based on the composition of the plagioclase. This reaction series displays a continuum from Ca-rich plagioclase (hot; dark-colored) to Na-rich plagioclase (cooler; light-colored). Ca is preferentially taken up in plagioclase crystallizing at high temperatures. As the melt(liquid) cools, the composition of this molten material is changing. Ca is being removed and incorporated into early-forming, high-temperature plagioclase crystals, leaving the molten component depleted in Ca but relatively enriched in Na. Thus plagioclase crystallized at lower temperatures is richer in Na. This reaction series is continuous, that is, even though the relative ratio of elements changes as the melt cools, the earlier-formed, high temperature crystals may still co-exist with later formed lower-temperature crystals. This is distinctly different from the discontinuous series, where the higher temperature crystals/minerals are generally destroyed in favor of the formation of the lower temperature crystals/minerals. Both the Continuous and Discontinuous Reaction Series occur simultaneously as a magma melt cools, thus giving rise to different mineral assemblages in rocks formed at different temperatures! So why are not all igneous rocks essentially composed of quartz?? Since it is on the bottom of the trend and represents the coolest (and therefore last) mineral to crystallize from a melt. This is primarily a function of the initial composition of the melt itself, and to a lesser degree, the initial temperature of the melt. Realistically, a melt does not go through the entire series. It starts out somewhere in the continuum and then typically goes through 3-4 minerals within the series before it becomes totally crystallized (it never makes it to the bottom). Also, keep in mind that if a melt is depleted in, or lacks a given element, lets say Ca for example, this will highly constrain the type of minerals that may crystallize out, regardless of temperature. Such a magma as suggested here (lacking Ca), could not form minerals such as Ca-rich plagioclase, pyroxenes, or amphiboles, because they all require some Ca in their structure. So the final rock formed would be composed of various proportions of the other minerals present, less those minerals that contain Ca. So how are porphyritic textures created in volcanic rocks? Bowens Reaction Series provides a ready means to explain how this texture may occur in volcanic rocks. By definition, a porphyritic texture means visible grains (crystals) within an invisible groundmass. Many people mistakenly call rocks that exhibit this texture as plutonic, because they can see individual grains. However, in a true plutonic rock, mineral grains are visible throughout the entire groundmass or matrix of the rock. Applying the principles of Bowens Reaction Series, as a melt begins to cool, specific minerals will begin to crystallize out (based on the temperature of the melt). If this melt is given the time and allowed to cool slowly, the entire melt will eventually crystallize into solid grains (minerals) that will be visible. If however, this same melt which has begun crystallizing out some of the minerals, is suddenly cooled (by reaching the surface of the Earth for example), the resulting rock will have frozen in it these visible minerals which had begun crystallizing (solidifying) out of the melt surrounded by a matrix (groundmass) of invisible minerals (your classic volcanic texture).
Potrebbero piacerti anche
- Geology Laboratory: Igneous Rocks and ProcessesDocumento10 pagineGeology Laboratory: Igneous Rocks and ProcessesIksan CahyadiNessuna valutazione finora
- Bowel's Reaction SeriesDocumento6 pagineBowel's Reaction SeriesAlfred StevenNessuna valutazione finora
- Bibliografia - Rochas Magmaticas Pamela GoreDocumento14 pagineBibliografia - Rochas Magmaticas Pamela GoreFilipa GuiaNessuna valutazione finora
- Unit 4 Lab Igneous Rocks SPR 24 Rev 4Documento20 pagineUnit 4 Lab Igneous Rocks SPR 24 Rev 4esoreaderNessuna valutazione finora
- Lab03 IgneousDocumento8 pagineLab03 Igneousgl8444893Nessuna valutazione finora
- Petrology 1Documento24 paginePetrology 1Rahul SharmaNessuna valutazione finora
- Petrology (1) 1 12Documento12 paginePetrology (1) 1 12Abhinav mothaNessuna valutazione finora
- Fractional MeltingDocumento19 pagineFractional MeltingShashankNessuna valutazione finora
- Mineral SilikatDocumento3 pagineMineral SilikatClara GintingNessuna valutazione finora
- Modul 5 Geolimia Dan Batuan BekuDocumento57 pagineModul 5 Geolimia Dan Batuan Bekurizkyade pratamaNessuna valutazione finora
- Types of Mineral DepositsDocumento11 pagineTypes of Mineral DepositsSayyad DawarNessuna valutazione finora
- Si Sio Fe Sio MG Sio Caco F CL BR I Caf Nacl S Zns PbsDocumento4 pagineSi Sio Fe Sio MG Sio Caco F CL BR I Caf Nacl S Zns PbsMiya GatotNessuna valutazione finora
- EES:1030 Igneous Rocks: September 6, 2018Documento5 pagineEES:1030 Igneous Rocks: September 6, 2018Kevin HoranNessuna valutazione finora
- Petrified Embryology Volume 6: The Frozen Baby Dinosaurs – Gryposaurus monumentensisDa EverandPetrified Embryology Volume 6: The Frozen Baby Dinosaurs – Gryposaurus monumentensisNessuna valutazione finora
- Magma CrytallizationDocumento6 pagineMagma CrytallizationDaring HunterzNessuna valutazione finora
- Igneous and Sedimentary RocksDocumento29 pagineIgneous and Sedimentary RocksgengkapakNessuna valutazione finora
- 07 Magmatism PDFDocumento8 pagine07 Magmatism PDFyahinnNessuna valutazione finora
- Classification of OresDocumento11 pagineClassification of OresDr R VishwanathNessuna valutazione finora
- The Bowen Reaction Series and Weathering of MineralsDocumento6 pagineThe Bowen Reaction Series and Weathering of Mineralsmichael100% (1)
- Classification of Mineral DepositsDocumento5 pagineClassification of Mineral DepositsMuhammad Sabeh Khan Panni100% (1)
- Earth Rocks (All Notes)Documento86 pagineEarth Rocks (All Notes)Rex TranquilliNessuna valutazione finora
- Batuan BekuDocumento49 pagineBatuan BekuAdistiNessuna valutazione finora
- 01 Igneous PETROLOGY NotesDocumento11 pagine01 Igneous PETROLOGY NotesdaanaahishmaelsNessuna valutazione finora
- Igneous Rocks & Their ActivitiesDocumento12 pagineIgneous Rocks & Their ActivitiesHemant Kumar SainiNessuna valutazione finora
- 10 Frequently Asked Questions About Rocks and RockDocumento13 pagine10 Frequently Asked Questions About Rocks and RockRocknCoolManNessuna valutazione finora
- 1940 Magmatic DifferentiationDocumento29 pagine1940 Magmatic Differentiationkhansa_161815100Nessuna valutazione finora
- Different Types of Rocks The Rock Cycle Properties and Classification of MineralsDocumento35 pagineDifferent Types of Rocks The Rock Cycle Properties and Classification of MineralschaibalinNessuna valutazione finora
- Classification of Mineral DepositsDocumento5 pagineClassification of Mineral DepositsJustin HernandezNessuna valutazione finora
- Earth Sci Igneous ProcessesDocumento31 pagineEarth Sci Igneous ProcessesJohn Rey DumaguinNessuna valutazione finora
- Cooling and TexturesDocumento4 pagineCooling and TexturesBroadsageNessuna valutazione finora
- المحاضرة الثالثةDocumento44 pagineالمحاضرة الثالثةfarrah.taNessuna valutazione finora
- Geo 14 Ebook Introduction To Mineralogy and Petrology 99 125Documento27 pagineGeo 14 Ebook Introduction To Mineralogy and Petrology 99 125Jose Elvis Ccalla OrucheNessuna valutazione finora
- Lecture 3.introduction To Ore Microscopy IIDocumento8 pagineLecture 3.introduction To Ore Microscopy IIDwi LekatompessyNessuna valutazione finora
- Chap05 Igneous RocksDocumento22 pagineChap05 Igneous RocksopulitheNessuna valutazione finora
- Metaminerals PDFDocumento8 pagineMetaminerals PDFPritam RajNessuna valutazione finora
- Rock CycleDocumento3 pagineRock Cycleapi-231516879Nessuna valutazione finora
- Bowens RS and Igneous Rock TexturesDocumento34 pagineBowens RS and Igneous Rock TextureshimeNessuna valutazione finora
- Chapter 3.1 - Igneous RockDocumento64 pagineChapter 3.1 - Igneous Rockalvinllp83Nessuna valutazione finora
- Lecture#03 04 Igneous RocksDocumento81 pagineLecture#03 04 Igneous RocksShahidulHoqueSohelNessuna valutazione finora
- Lab1 Rock Identification Rev01Documento26 pagineLab1 Rock Identification Rev01Anonymous FWTYseRvNessuna valutazione finora
- Igneous PDFDocumento78 pagineIgneous PDFStephen ErickNessuna valutazione finora
- Metamorphic, Igneous and Sedimentary Rocks : Sorting Them Out - Geology for Kids | Children's Earth Sciences BooksDa EverandMetamorphic, Igneous and Sedimentary Rocks : Sorting Them Out - Geology for Kids | Children's Earth Sciences BooksNessuna valutazione finora
- EARTHDocumento12 pagineEARTHNedy VillafuerteNessuna valutazione finora
- Hydrothermal Mineral DepositsDocumento12 pagineHydrothermal Mineral DepositsAdHy Fery SusantoNessuna valutazione finora
- Mineral Indeks Batuan MetamorfDocumento7 pagineMineral Indeks Batuan MetamorfReynara Davin Chen100% (1)
- ... Are And: Igneous RocksDocumento78 pagine... Are And: Igneous RocksTarek Mohamed100% (2)
- Endogenic Processes (Erosion and Deposition) : Group 3Documento12 pagineEndogenic Processes (Erosion and Deposition) : Group 3Ralph Lawrence C. PagaranNessuna valutazione finora
- Bowen: 'S Reaction SeriesDocumento4 pagineBowen: 'S Reaction SeriesAdzan RamadhanNessuna valutazione finora
- Group Three (Petrology)Documento7 pagineGroup Three (Petrology)thea4bermejoNessuna valutazione finora
- A Worksheet On Metamorphism and Rocks (Week 4)Documento9 pagineA Worksheet On Metamorphism and Rocks (Week 4)Ruth May-osNessuna valutazione finora
- Klasifikasi Endapan MineralDocumento4 pagineKlasifikasi Endapan MineralMull Mancunians PcgNessuna valutazione finora
- Earth Materials and ProcessesDocumento34 pagineEarth Materials and ProcessesJoanna Marie San JoseNessuna valutazione finora
- The Rock Cycle PDFDocumento3 pagineThe Rock Cycle PDFAlipAlBashri0% (1)
- Geology Study Guide One Through SevenDocumento13 pagineGeology Study Guide One Through SevenNikolaus Sean-Michael AlvaradoNessuna valutazione finora
- Bowens Reaction Series ExplanationDocumento3 pagineBowens Reaction Series ExplanationsuhantoroNessuna valutazione finora
- ESPGeology2 3Documento5 pagineESPGeology2 3ray mlkNessuna valutazione finora
- Classification of Mineral DepositsDocumento4 pagineClassification of Mineral DepositsWill PalomoNessuna valutazione finora
- 02 Handout Igneous RocksDocumento3 pagine02 Handout Igneous RocksEricson CecNessuna valutazione finora
- VC AndrewsDocumento3 pagineVC AndrewsLesa O'Leary100% (1)
- Waswere Going To Waswere Supposed ToDocumento2 pagineWaswere Going To Waswere Supposed ToMilena MilacicNessuna valutazione finora
- Corporate Restructuring Short NotesDocumento31 pagineCorporate Restructuring Short NotesSatwik Jain57% (7)
- DOST-PHIVOLCS Presentation For The CRDRRMC Meeting 15jan2020Documento36 pagineDOST-PHIVOLCS Presentation For The CRDRRMC Meeting 15jan2020RJay JacabanNessuna valutazione finora
- Case Study On Goodearth Financial Services LTDDocumento15 pagineCase Study On Goodearth Financial Services LTDEkta Luciferisious Sharma0% (1)
- D. Das and S. Doniach - Existence of A Bose Metal at T 0Documento15 pagineD. Das and S. Doniach - Existence of A Bose Metal at T 0ImaxSWNessuna valutazione finora
- Application of Contemporary Fibers in Apparel - LyocellDocumento5 pagineApplication of Contemporary Fibers in Apparel - LyocellVasant Kothari100% (1)
- Maharashtra State Board 9th STD History and Political Science Textbook EngDocumento106 pagineMaharashtra State Board 9th STD History and Political Science Textbook EngSomesh Kamad100% (2)
- Ose Sample QuotationDocumento37 pagineOse Sample Quotationrj medelNessuna valutazione finora
- Assessment 4 PDFDocumento10 pagineAssessment 4 PDFAboud Hawrechz MacalilayNessuna valutazione finora
- Steel Price Index PresentationDocumento12 pagineSteel Price Index PresentationAnuj SinghNessuna valutazione finora
- Jota - EtchDocumento3 pagineJota - EtchRidwan BaharumNessuna valutazione finora
- Windows Intrusion Detection ChecklistDocumento10 pagineWindows Intrusion Detection ChecklistJosé Tomás García CáceresNessuna valutazione finora
- KiSoft Sort & Pack Work Station (User Manual)Documento41 pagineKiSoft Sort & Pack Work Station (User Manual)Matthew RookeNessuna valutazione finora
- Contigency Plan On Class SuspensionDocumento4 pagineContigency Plan On Class SuspensionAnjaneth Balingit-PerezNessuna valutazione finora
- RevlonDocumento13 pagineRevlonSarosh AtaNessuna valutazione finora
- Introduction To Designing An Active Directory InfrastructureDocumento18 pagineIntroduction To Designing An Active Directory InfrastructurepablodoeNessuna valutazione finora
- T688 Series Instructions ManualDocumento14 pagineT688 Series Instructions ManualKittiwat WongsuwanNessuna valutazione finora
- XU-CSG Cabinet Minutes of Meeting - April 4Documento5 pagineXU-CSG Cabinet Minutes of Meeting - April 4Harold John LaborteNessuna valutazione finora
- Model Answer Winter 2015Documento38 pagineModel Answer Winter 2015Vivek MalwadeNessuna valutazione finora
- Four Hour Body Experiment Tracker TemplateDocumento4 pagineFour Hour Body Experiment Tracker Templatechanellekristyweaver100% (1)
- Book Speos 2023 R2 Users GuideDocumento843 pagineBook Speos 2023 R2 Users GuideCarlos RodriguesNessuna valutazione finora
- What Are The Advantages and Disadvantages of UsingDocumento4 pagineWhat Are The Advantages and Disadvantages of UsingJofet Mendiola88% (8)
- April 2021 BDA Case Study - GroupDocumento4 pagineApril 2021 BDA Case Study - GroupTinashe Chirume1Nessuna valutazione finora
- ICU General Admission Orders: OthersDocumento2 pagineICU General Admission Orders: OthersHANIMNessuna valutazione finora
- Principles To Action (Short)Documento6 paginePrinciples To Action (Short)nsadie34276Nessuna valutazione finora
- 16 Personalities ResultsDocumento9 pagine16 Personalities Resultsapi-605848036Nessuna valutazione finora
- Essay Final ProjectDocumento7 pagineEssay Final Projectapi-740591437Nessuna valutazione finora
- Cpar ReviewerDocumento6 pagineCpar ReviewerHana YeppeodaNessuna valutazione finora
- Simon Fraser University: Consent and Release FormDocumento1 paginaSimon Fraser University: Consent and Release FormpublicsqNessuna valutazione finora