Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Lorno HPLC
Caricato da
mostafaDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Lorno HPLC
Caricato da
mostafaCopyright:
Formati disponibili
International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol. 3, No.
3, pp 1220-1224, July-Sept 2011
Rapid RP-HPLC Method for Quantitative Determination of Lornoxicam in Bulk and Pharmaceutical Formulations
Aher K. B.1*, Bhavar G. B.1, Joshi H. P.2
1
Amrutvahini College of Pharmacy, Amrutnagar, Sangamner-422608, (M.S.), India. 2 IPDO, Dr. Reddys Laboratory, Hyderabad (A.P), India.
*Corres.author: aherkiran22@gmail.com
Abstract: A simple, rapid, and precise method has been developed for quantitative analysis of Lornoxicam (LXM) in pharmaceutical dosage forms. Chromatographic separation of LXM was achieved on a C18 analytical column with potassium dihydrogen phosphate buffer - acetonitrile, 70:30 (v/v), as mobile phase at ambient temperature. The flow rate was 1.0 ml/min and detection was by absorption at 291 nm using a photodiode-array detector. The number of theoretical plates and tailing factor for LXM were 6,577 and 1.03, respectively. The linearity of the method was excellent over the range 10100 g/ ml LXM. The correlation coefficient was 0.9999. Relative standard deviations of peak areas from six measurements were always less than 2%. The proposed method was found to be suitable and accurate for quantitative analysis of LXM. Keywords: Lornoxicam, RP-HPLC, method validation.
INTRODUCTION Lornoxicam (LXM, 6-chloro-4-hydroxy- 2-methyl-N2-pyridinyl-2H-thieno-[2,3-e]-1,2-thiazine-3carboxamide 1,1-dioxide; Figure 1) is a novel nonsteroidal anti-inflammatory drug (NSAID) in the enolic acid class of compound with analgesic, antiinflammatory and antipyretic properties [1, 2]. LXM, which is commercially available as an 8-mg tablet, is used to treat inflammatory diseases of the joints, osteoarthritis, pain after surgery, and sciatica. It works by blocking the action of cyclooxygenase, an enzyme involved in the production of chemicals, including some prostaglandins, in the body. All NSAIDs reduce inflammation caused by the bodys own immune system and are effective pain killers [3]. Methods for analysis of some oxicams by reversed-phase highperformance liquid chromatography (LC) [410], spectrofluorimetric and spectrophotometric methods using 7-chloro-4-nitrobenz- 2-oxa-1,3-diazole [11] and a voltammetric [12, 13] have been reported in the literature. A literature survey reveals that a spectrophotometric method has been used for analysis of LXM [14]; an LC method has been used for analysis of LXM and its metabolite in plasma and synovial fluid [15], and a liquid chromatographic electrospray ionization tandem mass spectrometric method [16] has also been used for analysis of LXM. The main purpose of the work presented was to develop simple, rapid, accurate, precise, linear, sensitive, robust and rugged HPLC method for the determination of LXM in bulk drug and pharmaceutical formulations.
Aher K. B.et al /Int.J. ChemTech Res.2011,3(3)
1221
Preparation of Potassium Dihydrogen Phosphate Buffer Accurately weighed 6.8 g of potassium dihydrogen phosphate was transferred to a 1000 mL volumetric flask, dissolved in Milli-Q water and volume adjusted up to the mark with the same solvent. Diluent preparation Mobile phase and acetonitrile were mixed in the ratio of 1:1 Preparation of standard stock solution and calibration curve Standard stock solution of concentration 100g/mL was prepared by dissolving 10 mg of LXM reference standard in diluent and diluting to 100 ml with the same solvent. Aliquots from the stock solution were diluted with the diluent to give the solutions in the concentration range 10-100 g/mL. The solutions were injected in HPLC and area was measured for each solution. The calibration curve was obtained by plotting peak area on ordinate against drug concentration on abscissa. Linear regression data is shown in table 1. Preparation of Sample solution for Assay Twenty tablets were weighed accurately and finely powdered. A powder equivalent to 10mg of LXM was transferred carefully to 100mL volumetric flask and about 30mL diluent was added. The mixture was sonicated for 10 minutes. The volume was made up to 100mL with diluent, filtered through whatman no. 5 filter paper. From the filtrate 10mL was pipetted out and diluted to 100mL with diluent. The final solution was injected in HPLC, chromatogram was recorded and area was measured. Results obtained are summarized in table 2. Table 1: Linear regression data of calibration graph Linearity range (g/mL) 10-100 Slope 37.16 Y-intercept 0.934 Correlation coefficient ( r) 0.9999 Table 2: Assay of LXM in tablets 8 Label claim (mg) Amount found (mg) SD 7.95 0.018 99.39 % % label claim 0.229 % RSD (n=6)
Figure 1: Structure of LXM EXPERIMENTAL Materials and reagents LXM reference standard was obtained from Themis Lab (Mumbai, India), acetonitrile (HPLC grade) from Merck, Potassium dihydrogen orthophosphate (KH2PO4) from Qualigens Fine Chemicals (Glaxo,Mumbai, India). The 0.45 m pump Nylon filter was obtained from Advanced Micro Devices (Ambala Cantt, India) & whatman no 5 filter paper was obtained from Modern Science lab, (Nashik, India). The drug product of LXM, i.e. Zion tablet (Unichem Laboratories, Mumbai, India) with a label claim of 8 mg drug was purchased commercially. Milli-Q water was used throughout the work. Other chemicals used were analytical or HPLC-grade and glasswares used were Class A grade. Equipment A gradient high-performance liquid chromatograph of an Agilent 1100 series instrument comprising of degasser, quaternary pump, auto injector, column compartment, and variable wavelength programmable UVVis detector was used. The system was controlled by Chemstation software. Analytical balance used for weighing was Make-Mettler Toledo, Model-XP 105. Sonarex Super RK 102 (35 KMZ) Bandelin (Berlin, Germany) equipment with thermostatically controlled heating (3080 C) was used for ultrasonication. Chromatographic conditions Chromatography was performed on Hypersil BDS C18 (200 X 4.6 mm, 5m particle) column (LGC Promochem, Banglore, India) at ambient temperature. The isocratic mobile phase was a 70:30(v/v) mixture of potassium dihydrogen phosphate buffer and acetonitrile at a flow rate of 1mL/min. the variable wavelength programmable UVVis detector was set at 291 nm.
Aher K. B.et al /Int.J. ChemTech Res.2011,3(3)
1222
Method validation
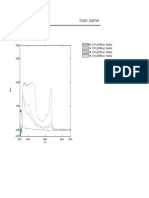
The developed method was validated as per ICH guidelines [17,18]. Linearity Linearity was studied in the concentration range of 10100g/mL. All measurements were repeated three times for each concentration. Correlation coefficient (r) of the line, constructed by plotting mean of peak areas against corresponding concentration, was found to be 0.9999. Specificity The optimized solvent system yielded a symmetric peak for the drug with Rt 8.184 min (Figure 2). The peak for the drug from tablets was identified by comparing the Rt and also comparing its absorbance spectrum with that obtained with the standard drug. Peak purity values were >999 for the drug product, which shows that the analyte peaks were pure and there were no interferences from formulation excipients in the analyte peak. Accuracy To ensure accuracy of the method, recovery studies were performed by standard addition method at 80%, 100% and 120% level to pre-analyzed samples and subsequent solutions were re-analyzed. At each level
three determinations were performed and the results obtained are shown in table 3. Precision Precision of the method was determined in terms of repeatability and intra-day and inter-day precisions. Repeatability of the method was determined by analyzing six samples of same concentrations of drug. Chromatographs were recorded and area of each chromatograph was measured. Results of this determination are reported in table 4. Intra day and Inter day Precision: Intra-day precision was determined by analyzing the drugs at three different concentrations and each concentration for three times, on the same day. Inter-day precision was determined similarly, but the analysis being carried out daily, for three consecutive days. The results are summarized in table 5. Ruggedness To determine ruggedness, two different analysts performed assay on marketed tablets of the drug in similar operational and environmental conditions using developed method. The results are summarized in table 6.
Table 3: Results of recovery studies Initial Amount recovered Excess drug Concentration added (g/mL) (g/mL SD) (g/mL) 10 8 7.95 0.081 10 10 10.02 0.015 10 12 12.09 0.035 Table 4: Repeatability of the method Concentration taken (g/mL) Concentration found (g/mLSD) % RSD (n=6) 10 9.97 0.025 0.255
% Recovery 99.37 100.18 100.79
% RSD (n=3) 1.021 0.154 0.287
Table 5: Results of intra-day and inter-day precision Intra-day precision Inter-day precision Concentration Concentration Concentration %RSD taken (g/mL) found found %RSD n=3 n=3 (g/mLSD) (g/mLSD) 10 0.263 0.217 9.94 0.026 9.970.022 40 90 39.82 0.084 89.93 0.341 0.212 0.380 39.940.089 89.690.373 0.222 0.416
Aher K. B.et al /Int.J. ChemTech Res.2011,3(3)
1223
Table 6: Results of ruggedness studies Parameter Analyst I 8 Label claim (mg) 7.97 Amount found (mg) 99.57 % Label claim 0.264 % RSD
Analyst II 8 7.98 99.76 0.224
Figure 2: Chromatogram of standard LXM
RESULTS AND DISCUSSION Several mob ile p has es wer e tried for the analysis. The mobile phase 70:30 (v/v) mixture of potassium dihydrogen phosphate buffer and Acetonitrile showed good resolution and good peak symmetry (Figure 2). Rt of LXM was found to be 8.184 min. Variable columns were used such as C18 (YMC, Alltima, and ACE) and CN (YMC and Alltima), a u Bondapack 5um (300 X 4.6 mm), Sherisorb ODS 5um (200 X 4.6). Hypersil 5um BDS C 18
(200 X4.6 mm) was selected for the analysis as it showed minimum elution time with good resolution. Linearity was observed in the concentration range of 10-100 g/mL, correlation coefficient (r) being 0.9999. The %RSD for intraday and inter-day precision was ranged between 0.212-0.380% and 0.217-0.416% respectively. The recoveries were ranged between 99.37-100.79%.
REFERENCES 1. 2. The Merck index, 2001, 13th ed. Merck, USA. Balfour JA, Fitton A and Barradell LB, Lornoxicam, A review of its pharmacology and therapeutic potential in the management of painful and inflammatory conditions, Drugs, 1996, 51 (4), 639657.
3.
4.
Radhofer-Welte S and Rabasseda X, Lornoxicam, a new potent NSAID with an improved tolerability profile, Drugs of Today, 2000, 36(1), 55-76. Wanwimolruk S., Wanwimolruk S. Z. and Zoset A.R., A simple and sensitive HPLC assay for Piroxicam in plasma and its application to bioavailability study, J. Liq.
Aher K. B.et al /Int.J. ChemTech Res.2011,3(3)
1224
5.
6.
7.
8.
9.
10.
11.
Chrom.& Rel.Technol., 1991, 14(12), 2373 2381. Owen S.G., Roberts M. S. and Frisen W. T., Rapid high-performance liquid chromato graphic assay for the simultaneous analysis of non-steroidal anti-inflammatory drugs in plasma, J. Chromatogr, 1987, 416(2), 293 302. Streete P. J., Rapid high-performance liquid chromatographic methods for the determination of overdose concentrations of some non-steroidal anti-inflammatory drugs in plasma or serum, J. Chromatogr, 1989, 495, 179193 Joseph-Charles J. and Bertucat M., Simultaneous high performance liquid chromatographic analysis of non-steroidal anti-inflammatory oxicams in pharmaceutical preparations, J. Liq. Chrom. & Rel. Technol., 2005, 22(13), 2009-2021. Cerretari D., Micheli L., Fiaschi A. I. and Giorgi G, Rapid and sensitive determination of Piroxicam in rat plasma, muscle and skin by high-performance liquid chromatography, J. Chromatogr. A, 1993, 614,103108. Mason J. L. and Hobbs G. J., Simple method for the analysis of tenoxicam in human plasma using high-performance liquid chromatography, J. Chromatogr. B:Analyt Technol Biomed Life Sci, 1995, 665(2), 410 415. Taha E. A., Salama N. N. and El-S Abdel Fattah, Stability-indicating chromatographic methods for the determination of some oxicams, J AOAC Int, 2004, 87(2),366373 Taha E. A., Salama N. N. and El-S Abdel Fattah, Spectrofluorimetric and Spectro photometric Stability-Indicating Methods for Determination of Some Oxicams Using 7-
12.
13.
14.
15.
16.
17.
18.
Chloro-4-nitrobenz-2-oxa-1,3-diazole (NBDCl), Chem Pharm Bull, 2006, 54(5), 653658. Bozal B. and Uslu B., Applications of carbon based electrodes for voltammetric determination of Lornoxicam in pharma ceutical dosage form and human serum, Combinatorial Chemistry & High Throughput Screening, 2010, 13, 599-609. Ghoneim M. M., Beltagi A. M. and Radi A., Wave adsorptive stripping voltammetric determination of the anti-infl ammatory drug Lornoxicam, Anal. Sci., 2002, 18(2), 183-186. Nemutlu E., Demircan S. and Kr S., Determination of Lornoxicam in pharmaceutical preparations by zero and first order derivative UV spectrophotometric methods, Pharmazie, 2005, 60 (6), 421-425 Radhofer-Welte S. and Dittrich P., Determination of the novel nonsteroidal antiinfl ammatory drug Lornoxicam and its main metabolite in plasma and synovial fluid, J. Chromatogr. B:Analyt Technol Biomed Life Sci, 1998, 707, 151159. Kim Y.H., Ji H.Y., Park E. S., Chae S. W. and Lee H. S., Liquid chromatographyelectrospray ionization tandem mass spectrometric determination of Lornoxicam in human plasma, Arch Pharm Res, 2007, 30(7), 905910. International Conference on Harmonisation (1996) Guidelines for the photostability testing of new drug substances and products, step 4, Q1B. International Conference on Harmonisation (2005) Validation of analytical procedures: text and methodology. In ICH Harmonized Tripartite Guidelines Q2 (R1), November 2005.
*****
Potrebbero piacerti anche
- HerbalismDocumento26 pagineHerbalismalejandro jeanNessuna valutazione finora
- Topical Gel Formulations PDFDocumento3 pagineTopical Gel Formulations PDFnofaliasariNessuna valutazione finora
- مذكرة فارماكولوجي روعةDocumento56 pagineمذكرة فارماكولوجي روعةKomang Gede Suwija Negara100% (1)
- Teva PharmaceuticalDocumento17 pagineTeva PharmaceuticalGanesh VedhachalamNessuna valutazione finora
- Why Is PH Important For HPLC BuffersDocumento4 pagineWhy Is PH Important For HPLC BuffersmostafaNessuna valutazione finora
- Experimental approaches to Biopharmaceutics and PharmacokineticsDa EverandExperimental approaches to Biopharmaceutics and PharmacokineticsNessuna valutazione finora
- 800 Questions PDF For MobileDocumento135 pagine800 Questions PDF For MobileImmad AlviNessuna valutazione finora
- 10 bad HPLC mobile phase habits to avoidDocumento2 pagine10 bad HPLC mobile phase habits to avoidmostafaNessuna valutazione finora
- 10 bad HPLC mobile phase habits to avoidDocumento2 pagine10 bad HPLC mobile phase habits to avoidmostafaNessuna valutazione finora
- A Comparative Study For The Quantitative Determination of Paracetamol in Tablets Using UVDocumento7 pagineA Comparative Study For The Quantitative Determination of Paracetamol in Tablets Using UVRizqita Atikah SNessuna valutazione finora
- Cosmeticsfornail PDFDocumento31 pagineCosmeticsfornail PDFRosanella Galindo100% (1)
- Drug Stability and KineticsDocumento29 pagineDrug Stability and Kineticsnovi_lingga67% (12)
- Karl Fisher ManuelDocumento80 pagineKarl Fisher ManuelpajovicmNessuna valutazione finora
- Koçak Farma 2021اصنافDocumento22 pagineKoçak Farma 2021اصنافدطه الصمدي100% (1)
- Indian Journal of Research in Pharmacy and BiotechnologyDocumento144 pagineIndian Journal of Research in Pharmacy and BiotechnologyDebjit Bhowmik0% (1)
- Validated RP-HPLC Method For Analysis of Aripiprazole in A FormulationDocumento6 pagineValidated RP-HPLC Method For Analysis of Aripiprazole in A Formulationblashyrkh_79Nessuna valutazione finora
- Development_and_validation_of_a_reversed (1)Documento14 pagineDevelopment_and_validation_of_a_reversed (1)Karina Guadarrama HernándezNessuna valutazione finora
- A Validated RP-HPLC Method For The Determination of Celecoxib in Bulk and Pharmaceutical Dosage FormDocumento5 pagineA Validated RP-HPLC Method For The Determination of Celecoxib in Bulk and Pharmaceutical Dosage FormOskar LazaroNessuna valutazione finora
- 15 Ac19Documento15 pagine15 Ac19Dana StoinNessuna valutazione finora
- DiacereinDocumento6 pagineDiacereinRikin ShahNessuna valutazione finora
- Method Development and Validation of Paracetamol Drug by RP-HPLC 1Documento7 pagineMethod Development and Validation of Paracetamol Drug by RP-HPLC 1Anonymous ncDgoMONessuna valutazione finora
- UV method for metformin quantification in tabletsDocumento4 pagineUV method for metformin quantification in tabletsWilliam SmithNessuna valutazione finora
- New RP-HPLC Method For The Determination of Olmesartan Medoxomil in Tablet Dosage FormDocumento7 pagineNew RP-HPLC Method For The Determination of Olmesartan Medoxomil in Tablet Dosage FormsanjeevbhatNessuna valutazione finora
- Development and Validation of HPLC Method For The Estimation of Nicergoline in Marketed FormulationsDocumento5 pagineDevelopment and Validation of HPLC Method For The Estimation of Nicergoline in Marketed FormulationsRatnakaram Venkata NadhNessuna valutazione finora
- Cephalexine Spectro VisDocumento0 pagineCephalexine Spectro VisVictor Purnama Agung FanggidaeNessuna valutazione finora
- HPLC Validation of Carbamazepine CR TabletsDocumento2 pagineHPLC Validation of Carbamazepine CR Tabletsأحمد الجيارNessuna valutazione finora
- Nadifloxacin - HPTLC Stability Indicating PDFDocumento8 pagineNadifloxacin - HPTLC Stability Indicating PDFNájla KassabNessuna valutazione finora
- Jurnal HPLCDocumento3 pagineJurnal HPLCRiche Dewata S.Nessuna valutazione finora
- Simultaneous Determination of Cefotaxime Sodium and Paracetamol by LC-MSDocumento7 pagineSimultaneous Determination of Cefotaxime Sodium and Paracetamol by LC-MSIOSR Journal of PharmacyNessuna valutazione finora
- Development and Validation of A LC/MS/MS Method For The Determination of Duloxetine in Human Plasma and Its Application To Pharmacokinetic StudyDocumento14 pagineDevelopment and Validation of A LC/MS/MS Method For The Determination of Duloxetine in Human Plasma and Its Application To Pharmacokinetic StudyMohamed Medhat AliNessuna valutazione finora
- 1 s2.0 S0165022X05001119 MainDocumento14 pagine1 s2.0 S0165022X05001119 MainBivin EbenezerNessuna valutazione finora
- Validation of UV Spectrophotometric Method For Determination of AtenololDocumento4 pagineValidation of UV Spectrophotometric Method For Determination of AtenololElfiaNeswitaNessuna valutazione finora
- Martins 2019Documento7 pagineMartins 2019Hasna NoerNessuna valutazione finora
- Simultaneous Estimation of Ibuprofen and Famotidine in Pure and Combination Dosage Form by RP-HPLCDocumento5 pagineSimultaneous Estimation of Ibuprofen and Famotidine in Pure and Combination Dosage Form by RP-HPLCrajj_2323Nessuna valutazione finora
- 4 DPC-2011-3-6-494-499Documento6 pagine4 DPC-2011-3-6-494-499Quty Papa KannaNessuna valutazione finora
- Development and Validation of A Simple HPLC Method For Simultaneous in Vitro Determination of Amoxicillin and Metronidazole at Single WavelengthDocumento5 pagineDevelopment and Validation of A Simple HPLC Method For Simultaneous in Vitro Determination of Amoxicillin and Metronidazole at Single WavelengthAnkit VishnoiNessuna valutazione finora
- Art 04Documento6 pagineArt 04sportcar2000Nessuna valutazione finora
- IbandronateDocumento6 pagineIbandronateAashishThakurNessuna valutazione finora
- Stability Indicating RP-HPLC Method for Drug EstimationDocumento15 pagineStability Indicating RP-HPLC Method for Drug EstimationAfonso RobertoNessuna valutazione finora
- RP-HPLC Method for Determining AlprazolamDocumento7 pagineRP-HPLC Method for Determining AlprazolamErwin FernándezNessuna valutazione finora
- 191 379 1 SMDocumento6 pagine191 379 1 SMPravin LondheNessuna valutazione finora
- صفوان عاشورDocumento8 pagineصفوان عاشورSudhanshu SinghNessuna valutazione finora
- Reverse Phase HPLC Method For Determination of Aceclofenac and Paracetamol in Tablet Dosage FormDocumento4 pagineReverse Phase HPLC Method For Determination of Aceclofenac and Paracetamol in Tablet Dosage FormNguyễn TrinhNessuna valutazione finora
- Validated RP - HPLC Method For The Estimation of Liraglutide in Tablet DosageDocumento10 pagineValidated RP - HPLC Method For The Estimation of Liraglutide in Tablet DosageInternational Journal of Science Inventions TodayNessuna valutazione finora
- 213 SharmaDocumento4 pagine213 SharmaFaradies ArijaNessuna valutazione finora
- HPLC METHOD VALIDATIONDocumento5 pagineHPLC METHOD VALIDATIONbavirisettikiranNessuna valutazione finora
- New RP-HPLC Method for Orlistat AnalysisDocumento7 pagineNew RP-HPLC Method for Orlistat AnalysisEduardo BarreraNessuna valutazione finora
- Estimation of Meropenem in Human Plasma by HPLC-UV and Its Application in Comparative Bioavailability StudyDocumento8 pagineEstimation of Meropenem in Human Plasma by HPLC-UV and Its Application in Comparative Bioavailability Studyayand2005Nessuna valutazione finora
- Development and Validation of Stability Indicating Assay Method For Simultaneous Estimation of Amoxicillin Trihydrate and Cloxacillin Sodium in Pharmaceutical Dosage Form by Using RP-HPLCDocumento13 pagineDevelopment and Validation of Stability Indicating Assay Method For Simultaneous Estimation of Amoxicillin Trihydrate and Cloxacillin Sodium in Pharmaceutical Dosage Form by Using RP-HPLCJermy ErmiNessuna valutazione finora
- Articol AlprazolamDocumento4 pagineArticol AlprazolamJeffrey HaleNessuna valutazione finora
- Journal of Chemical and Pharmaceutical Research, 2013, 5 (5) :1-11Documento11 pagineJournal of Chemical and Pharmaceutical Research, 2013, 5 (5) :1-11NurulnameiiNessuna valutazione finora
- Procedure Jurnal AtenololDocumento3 pagineProcedure Jurnal AtenololNining KurniasihNessuna valutazione finora
- Extractive Spectrophotometric Determination of Nicergoline Through Ion-Pair Complexation ReactionDocumento7 pagineExtractive Spectrophotometric Determination of Nicergoline Through Ion-Pair Complexation ReactionHeidi HughesNessuna valutazione finora
- 20211226124933a5 64 JCM 2108 2174Documento11 pagine20211226124933a5 64 JCM 2108 2174Venkat PalaganiNessuna valutazione finora
- Development and Validation of Reversed-Phase HPLC Method For Simultaneous Estimation of Rosuvastatin and Fenofibrate in Tablet Dosage FormDocumento6 pagineDevelopment and Validation of Reversed-Phase HPLC Method For Simultaneous Estimation of Rosuvastatin and Fenofibrate in Tablet Dosage FormshraddhaJPNessuna valutazione finora
- HPLC Research PaperDocumento19 pagineHPLC Research PaperMit PatelNessuna valutazione finora
- Analytical Method Development and Validation of Minoxidil in Pharmaceutical Dosage Forms by UV SpectrophotometryDocumento4 pagineAnalytical Method Development and Validation of Minoxidil in Pharmaceutical Dosage Forms by UV SpectrophotometryBil Sonador100% (1)
- 7.meto Hydro TLCDocumento6 pagine7.meto Hydro TLCBaru Chandrasekhar RaoNessuna valutazione finora
- OutDocumento11 pagineOutAngel EivinoNessuna valutazione finora
- Degradation PramipexoleDocumento9 pagineDegradation Pramipexoleclaudiamaniac7Nessuna valutazione finora
- Scholars Research Library: Madhukar. A, K. Naresh, CH. Naveen Kumar, N. Sandhya and P. PrasannaDocumento5 pagineScholars Research Library: Madhukar. A, K. Naresh, CH. Naveen Kumar, N. Sandhya and P. PrasannaQuty Papa KannaNessuna valutazione finora
- PCM PhenergenDocumento4 paginePCM PhenergenYoobNorismawandiNessuna valutazione finora
- RP-HPLC Method Development and Validation For The Estimation of Diclofenac Sodium, Tramadol Hydrochloride and Chlorzoxazone From Their Combined Tablet Dosage FormDocumento6 pagineRP-HPLC Method Development and Validation For The Estimation of Diclofenac Sodium, Tramadol Hydrochloride and Chlorzoxazone From Their Combined Tablet Dosage FormPinak PatelNessuna valutazione finora
- IJRPBSDocumento8 pagineIJRPBSrakesh2284Nessuna valutazione finora
- Validated UV method for Amlodipine, Valsartan and HydrochlorothiazideDocumento5 pagineValidated UV method for Amlodipine, Valsartan and Hydrochlorothiazideabdelaziz_ismail685662Nessuna valutazione finora
- Spectrophotometric Determination of Cardiovascular DrugsDocumento7 pagineSpectrophotometric Determination of Cardiovascular DrugsIJMERNessuna valutazione finora
- Stability-indicating RP-HPLC method for racecadotril quantificationDocumento8 pagineStability-indicating RP-HPLC method for racecadotril quantificationAnjay MalikNessuna valutazione finora
- Analytical Method Development and Validation of Caffeine in Tablet Dosage Form by Using UV-SpectrosDocumento5 pagineAnalytical Method Development and Validation of Caffeine in Tablet Dosage Form by Using UV-SpectrosKrisna Raditya PNessuna valutazione finora
- Food Safety: Innovative Analytical Tools for Safety AssessmentDa EverandFood Safety: Innovative Analytical Tools for Safety AssessmentUmile Gianfranco SpizzirriNessuna valutazione finora
- Practical Handbook of Pharmaceutical Chemistry for M.PharmDa EverandPractical Handbook of Pharmaceutical Chemistry for M.PharmNessuna valutazione finora
- Mobile Phase EffectDocumento3 pagineMobile Phase EffectmostafaNessuna valutazione finora
- Biopharmaceutics Classification System BCS-based BDocumento4 pagineBiopharmaceutics Classification System BCS-based BmostafaNessuna valutazione finora
- Strategies To Address Low Drug Solubility PDFDocumento185 pagineStrategies To Address Low Drug Solubility PDFRodrigo AvilaNessuna valutazione finora
- Photometric Report: File Name: F:/CPH/Pre 1-8 Not Dil - PhoDocumento2 paginePhotometric Report: File Name: F:/CPH/Pre 1-8 Not Dil - PhomostafaNessuna valutazione finora
- Dissolution Method Development and ValidDocumento7 pagineDissolution Method Development and ValidmostafaNessuna valutazione finora
- Importance of Controlling Mobile Phase pH in RP HPLCDocumento7 pagineImportance of Controlling Mobile Phase pH in RP HPLCAbel Jipsonx Pardo BqueNessuna valutazione finora
- Writing Successfully in Science by Maeve OConnorDocumento241 pagineWriting Successfully in Science by Maeve OConnormostafaNessuna valutazione finora
- Cleaning Validation MACO v2 0Documento2 pagineCleaning Validation MACO v2 0mostafaNessuna valutazione finora
- Reverse Phase HPLC of Ionizable SamplesDocumento4 pagineReverse Phase HPLC of Ionizable SamplesmostafaNessuna valutazione finora
- Buffer Choice For HPLC SeparationsDocumento3 pagineBuffer Choice For HPLC SeparationsmostafaNessuna valutazione finora
- Effectiveness of Bisphosphonates On Nonvertebral and Hip Fracture Ris and Alendro198 - 2006 - Article - 274Documento10 pagineEffectiveness of Bisphosphonates On Nonvertebral and Hip Fracture Ris and Alendro198 - 2006 - Article - 274mostafaNessuna valutazione finora
- 10.1007 s00198 012 2175 7 PDFDocumento10 pagine10.1007 s00198 012 2175 7 PDFmostafaNessuna valutazione finora
- PIO Volume10 3Documento79 paginePIO Volume10 3mostafaNessuna valutazione finora
- Qualification of Equipment: Bin Blender and Compression MachineDocumento5 pagineQualification of Equipment: Bin Blender and Compression MachineMusab HashmiNessuna valutazione finora
- Fluconazole TabletsDocumento2 pagineFluconazole TabletsmostafaNessuna valutazione finora
- Validation For The DeterminationDocumento18 pagineValidation For The DeterminationmostafaNessuna valutazione finora
- LC US Pharmacopeia MethodsDocumento5 pagineLC US Pharmacopeia MethodsmostafaNessuna valutazione finora
- Zorbax L Number Eq Pharma PDFDocumento2 pagineZorbax L Number Eq Pharma PDFmostafaNessuna valutazione finora
- Estimation of Shelf Life For Water-Based Paints Using Regression MethodsDocumento11 pagineEstimation of Shelf Life For Water-Based Paints Using Regression MethodsmostafaNessuna valutazione finora
- Validation For The DeterminationDocumento18 pagineValidation For The DeterminationmostafaNessuna valutazione finora
- 634702538270340000Documento34 pagine634702538270340000mostafaNessuna valutazione finora
- WatercressDocumento7 pagineWatercressmostafaNessuna valutazione finora
- 3191 11907 1 PBDocumento5 pagine3191 11907 1 PBmostafaNessuna valutazione finora
- Water Stills Brochure GFLDocumento19 pagineWater Stills Brochure GFLFelix SelorioNessuna valutazione finora
- Indomethacin - Drug InformationDocumento16 pagineIndomethacin - Drug InformationAbraham SarabiaNessuna valutazione finora
- Moist Heat Sterilization Validation and Requalification STERISDocumento4 pagineMoist Heat Sterilization Validation and Requalification STERISDany RobinNessuna valutazione finora
- Mechanism of Action of Ariprazole PDFDocumento5 pagineMechanism of Action of Ariprazole PDFDio PattersonNessuna valutazione finora
- Definition and Sources of DrugsDocumento29 pagineDefinition and Sources of DrugsSAHILA SHEIKHNessuna valutazione finora
- January 25, 2010 IDocumento75 pagineJanuary 25, 2010 IomairfarooqNessuna valutazione finora
- RKO bulan juni apotek Vanisa obat penjualan dan persediaanDocumento10 pagineRKO bulan juni apotek Vanisa obat penjualan dan persediaanressamahendraNessuna valutazione finora
- Vincristine (OncovinDocumento4 pagineVincristine (Oncovin9959101161Nessuna valutazione finora
- Glycans AplicacionesDocumento180 pagineGlycans AplicacionesIvanaColinNessuna valutazione finora
- The CFO-HR HarmonyDocumento48 pagineThe CFO-HR Harmonyusf69Nessuna valutazione finora
- Case StudyDocumento3 pagineCase StudyAngelica Joyce SinnacoNessuna valutazione finora
- Lesson 6 (New) Medication History InterviewDocumento6 pagineLesson 6 (New) Medication History InterviewVincent Joshua TriboNessuna valutazione finora
- Antibacterial ChemotherapyDocumento47 pagineAntibacterial ChemotherapyPawan PatelNessuna valutazione finora
- Form 19-CDocumento15 pagineForm 19-Cs.sabapathyNessuna valutazione finora
- Restrictions in Use and Availability of PharmaceuticalsDocumento328 pagineRestrictions in Use and Availability of PharmaceuticalsinfooncoNessuna valutazione finora
- Wellness RevolutionDocumento4 pagineWellness RevolutionWaqar GhoryNessuna valutazione finora
- Ranbaxy Nigeria Limited Product CatalogDocumento2 pagineRanbaxy Nigeria Limited Product CatalogikponmwonsaNessuna valutazione finora
- AnnexD.2 CertificationOfServiceDeliverySupport (Medicines)Documento3 pagineAnnexD.2 CertificationOfServiceDeliverySupport (Medicines)Arlyn DapulaNessuna valutazione finora
- Loxapine PDFDocumento2 pagineLoxapine PDFDavid AdamsNessuna valutazione finora
- InventoryDocumento12 pagineInventoryMarko ParungoNessuna valutazione finora
- ANCOVA Approach For Premarketing Shelf Life Determination With Multiple FactorsDocumento6 pagineANCOVA Approach For Premarketing Shelf Life Determination With Multiple FactorsEcaterina AdascaliteiNessuna valutazione finora
- SennaDocumento4 pagineSennaMohamed FadlNessuna valutazione finora
- Daftar PustakaDocumento6 pagineDaftar PustakaummiheryanaNessuna valutazione finora
- 2 Marks QuestionsDocumento6 pagine2 Marks QuestionsAdnanNessuna valutazione finora
- Dermovate™ 0.05% W/W Ointment: Package Leaflet: Information For The UserDocumento6 pagineDermovate™ 0.05% W/W Ointment: Package Leaflet: Information For The UsermahardiantooNessuna valutazione finora