Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Urine and Blood PPT (Handout Print Form) Chua, R
Caricato da
Kirsten Hazel MejiaDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Urine and Blood PPT (Handout Print Form) Chua, R
Caricato da
Kirsten Hazel MejiaCopyright:
Formati disponibili
3/9/2011
URINE
Sterile, liquid by-product of the body that is secreted by the kidneys. Cellular metabolism generates numerous by-products that require elimination from the bloodstream. These by-products are eventually expelled from the body in a process known as micturition.
Activity 26: Physical Properties of Urine
COLOR straw to amber *Colorless to straw indicates low specific gravity and large quantity *Amber indicates high specific gravity and small quantity
ODOR aromatic - depends on diet and drugs used Cloudiness: -freshly voided is clear (usually) but cloudy sometimes due to the following sediments: Amorphous phosphates form white cloud/precipitate Amorphous urates form a white/pink cloud of sediment Epithelial cells and mucus when large in amounts Pus makes urine turbid Fat render urine turbid Bacteria - produce uniform cloudiness
Abnormal (Pathological Coloration) Reddish amber increased in urobilinogen Brownish yellow/green indicate bile pigments Red to smoky brown due to blood and blood pigments Milky due to large amounts of pus, bacteria/fat Brownish black indicate melanin
pH -4.8-7.5 (Average: 6) 24 Hr. Specimen are less acidic than freshly passed and may become alkaline after standing Pathological Significance: Acidity - increased in acidosis, fevers, diet with excess proteins Alkaline in chronic cystitis and urine retention due to decomposition of urine in bladder
SPECIFIC GRAVITY (24 Hr. Specimen) -1.015 to 1.025 COMPOSITION -H2O: about 1000-1500g -Solids: about 55-70g in 24 hours Nitrogenous organic compounds Non-nitrogenous organic substances Inorganic salts Traces of Fe, Cu, Zn, Cl..etc.
3/9/2011
Average volume: 1-2 L a day
1.
Why does urine become turbid on standing? flocculate of molecules composed of nucleoprotein mucoid precipitation of calcium phosphate decomposition of urea to form ammonia fat globules or pus cells infection in the urinary tract
ABNORMAL
Polyuria = a condition of excessive production of urine Oliguria = decreased in quantity Anuria = total suppression of urine
2. Explain the alkaline fermentation of urine.
-
3. What is the importance of determining the specific gravity of urine? rough estimate of the total solids in the urine provide information of the state of hydration of the patient - if highly concentrated, the patient is dehydrated determine the presence of renal disease helps distinguish between renal failure and dehydration determines the concentrating ability of the kidneys
Ammoniacal fermentation the conversion of the urea of the urine into ammonium carbonate, through the growth of bacteria. CON2H4 + 2H2O (NH4)2CO3 Note: Whenever urine is exposed to the air in open vessels for several days it undergoes this alkaline fermentation.
4. Why must the examination be done on a 24-hour specimen? if the complete examination of the constituents of the urine is needed notably better than that exceeding 24-hours because: - microorganisms may grow - convert urea to ammonium carbonate giving a more pungent ammoniacal odor than normal - nitrogenous constituents are altered by the loss of nitrogen as volatile ammonia
5.
What are the substances responsible for the normal color of urine? urochrome gives the normal yellow color is due to a pigment metabolite arising from the body's destruction of hemoglobin. uroerythrin urinary pigment that gives a redish yellow color to deposits of urates. urobilinogen when oxidized gives a darker or deeper yellow color
3/9/2011
6. Outline the origin of the color of urine and feces.
Hemoglobin Heme Biliverdin + 2H (reduction) Bilirubin +2H Mesobilirubinogen D-Urobilinogen +2H -2H
Stercobilinogen (L-urobilinogen)
+ Protein (Globin)
Activity 27: Constituents of Urine
Urobilin
-2H (auto-oxidation) D-Urobilin
-2H
Stercobilin (L-urobilin)
A.1. Sulfate white ppt. A.2. Chloride white ppt. A.3.b. Alkali Phosphate white ppt.
1 Urine Constituents
3.b.
What is triple phosphate?
- Ammonium magnesium phosphate
A.4.a. Addition of 1 ml. of ammonium thiocyanate - Formation of red color A.4.b. With 5 drops of potassium ferrocyanide - Blue solution
Sources of phosphate in urine: - breakdown of tissue, foods rich in phosphoproteins, phospholipids and nucleoproteins
3.a. Triple Phosphate
3/9/2011
How does urea become a component of urine?
It is the pricipal and product of protein metabolism and constitutes about 80% - 90% total nitrogen excretion.
B.1. Urea Crystals
B.2.a. Uric Acid Crystals
B.2.b. Result: Formation of Black spot Uric acid - Reducing property How does it become a component of urine?
-Uric acid is the final end product of purine oxidation in the body
3.a. Weyls Reaction Formation of Red color
3.b. Jaffes Reaction Dark orange - red solution
How does creatinine become a component of urine? - As a product from metabolism in the muscle tissues.
How does it become a component of urine?
- C8H6NOSOK -The potassium salt of indoxylsulfate (indican) found in urine is a result of bacterial action on tryptophan in the bowel.
4. Obermeyers Test blue color
5.a. Hippuric Acid Crystals
Odor:
6. Oxalic Acid Crystals
Irritating odor
3/9/2011
C.PATHOLOGICAL
Result brick red ppt. White ring b/n two liquids Red purple Blue green color
Acetone Albumin
7. Pigments Result White ppt. What pigments are usually present in urine?
Glucose brick red ppt.
1 . Glucose 2. Albumin
(Hellers Test) white ring
- Urochrome, urobilinogen, and uroerythrin
3. Acetone
8. Purine Bases Cloudy soln w/ white ppt.
Blood blue green color
4. Blood
(Legals Test) Red purple
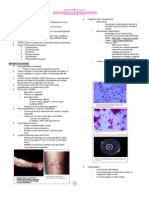
What are the different microscopic constituents of urine?
Uric Acid
Calcium oxalate
Hippuric Acid
Ammoniummagnesium phosphate
Calcium phosphate
Cystine
Tyrosine
Cholesterol
Epithelial cells
Epithelial cells
Squamous cells
Leukocytes
D. Uric Crystals
Hyaline casts
Granular casts
Granular casts
Epithelial casts
Pus casts
Blood casts
Mucus threads
Crystals
Cells and Casts
Questions: 1. What pathological condition is indicated by? a. Glucose -normally cannot be detected in urine -its presence in appreciable amount is termed as glycosuria -alimentary glycosuria (glycuresis) occurs after intake of CHO -pathological conditions of the kidney such as glomerulonepthritis -increased amount of glucose in blood is called hyperglycemia -hyperglycemia and glycosuria is found in: diabetes mellitus b. Albumin its presence is called albuminuria; properly termed as proteinuria. 2 types: Functional - no diseased organ and the excretion is only slight and temporary due to strenuous exercises, cold baths, fever Pathological maybe either renal, pre-renal and post renal c. Ketones (end product of fatty acid metabolism) in urine made up of: -Aceto-acetic acid -Butyric acid -Hydroxybutyric acid -Acetone the first three are normal products of fat metabolism they are produced in the liver are utilized or destroyed in the extrahepatic cells **as long as production compensates destruction, no Ketonuria is produced; but when production is too great- ketonemia then ketonuria develops d. Blood its presence is called hematuria. Found whenever there is lesion in the kidney or any part of the urinary tract Hemoglobinuria presence of hemoglobin in urine due to: destruction of RBC liver cannot change all the hemoglobin into bile pigments
3/9/2011
2. What is meant by creatinine coefficient and what is its significance? Creatinine the anhydride of creatine creatinine bears a direct relation to the muscle mass of the individual expressed as CREATININE COEFFICIENT (amount of creatinine in mg excreted in 24 hours per kg of body weight) average adult male coefficient is 20 26; female is 14 22 ** Significance: decrease value indicates muscle wasting due to prolonged negative nitrogen balance; seen in starvation, diabetes, muscle dystrophy
3. Why is lactose found in urine of lactating women? Lactosuria appears after the 3rd month of pregnancy and increases in incidence up to the 35th week and following delivery -chiefly of mammary origin even prior to lactation -caused by high level of lactose of mammary origin in the maternalhoodstage -lactose is excreted by glomerular filtration and is not reabsorbed by the tubules so that all the filtered lactose appears in the urine
4. What is the significance of large amounts of indican in urine? Indican salts of indoxyl sulfate derived from indole which in turn arises from the action of putrefying bacteria on tryptophan and CHONS containing it. -occurs in large intestines if any indole is absorbed -it undergoes a series of detoxification transformations in the liver and indoxyl is formed -it is conjugated with sulfate and neutralized to yield a salt -indican is eliminated in the urine and can be detected by OBERMEYERS TEST -an increase in the amount is indicative of increased putrefaction in the large intestines
5. What is phosphaturia and is its significance? -normally kidneys excrete between 1 and 5 grams of phosphoric acid in the form of phosphates but at times it becomes excessive . Such abnormal excretion and precipitation is called PHOSPHATURIA 2 types of phosphate excreted: a. Alkaline phosphate those combined with alkali metals like Na, K, and NH4+ b. Earthy phosphate combined with earth metals like Ca, Mg SIGINFICANCE: -the amount is increased in hyperthyroidism -in the early stages of pulmonary tuberculosis -diseases on the bones like rickets/osteomalacia -index for the extent of phosphate metabolism
BLOOD
Activity 28: Substances in Blood
Specialized bodily fluid that delivers necessary substances to the body's cells such as nutrients and oxygen and transports waste products away from those same cells
3/9/2011
Erythrocytes (Red Blood Cells) -7-8um diameter -Biconcave disc -without nuclei -lifespan: 120 days
Blood performs many important functions within the body: Supply of oxygen to tissues Supply of nutrients such as glucose, amino acids, and fatty acids Removal of waste such as carbon dioxide, urea, and lactic acid Immunological functions, including circulation of white blood cells, and detection of foreign material by antibodies Coagulation Regulation of body pH Regulation of core body temperature
Leukocytes (White Blood Cells) -Have nucleus -Most live for a few hours to a few days
Thrombocytes (Platelets) -2-4 um diameter -no nucleus -Lifespan: 5-9 days
A. Inorganic substances 1. Reagent: Ash + dil. HCl + potassium ferrocyanide Result: prussian blue solution Constituent of blood present: iron Equation: 4Fe+3 + 3Fe(CN) -4 6 Fe4[Fe(CN)6]3 2. Reagent: blood + water + heat to boiling Result: redish brown solution Reagent: dil. HNO3 + AgNO3 Result: white ppt. formed Constituent of blood present: chloride Equation: AgNO3 + Cl AgCl + NO3
3. Reagent: dil. HNO3 + ammonium molybdate Result: formation of yellow brown ppt. Constituent of blood present: phosphate Equation: H2PO4 + 12MoO4 + 3NH4 + 22H3O (NH4)3PO4.12MoO4 + 34H2O 4. Reagent: ammonium oxalate Result: formation of white ppt Constituent of blood present: calcium Equation: Ca+2 + C2O4 CaC2O4
B. Hemoglobin Blood exposed to air: dark red color Conclusion: formation of oxyhemoglobin took place upon exposure of blood to air C. Glucose in Blood occurs after the separation from coagulated proteins Reagent: Benedicts reagent Result: brick red ppt.
3/9/2011
Questions:
1.
2. Structure of the Hemoglobin molecule conjugated CHON with a Mw of 68000 made up of CHON globin to which heme groups are attached globin contains high amount of histidine, lysine and little of isoleucine (for the specificity of hemoglobin) its buffering property and the CO2 carrying capacity iron containing pigment heme is specially for the pigmentary property
Inorganic constituents of blood and its function Calcium plays a role in blood clotting Phosphorus for the maintenance of c id-base balance and calcium equilibrium Chlorine in the form of NaCl- plays a role in osmotic pressure Magnesium 18 parts magnesium gives rise in rate vasodilation and hyperirritability of the nervous system Iron essential component of hemoglobin, muscle myoglobin Copper important in bone formation and cellular respiration
3. Methemoglobin and its color a derivative in which iron is in the ferric state produced by the oxidation of hemoglobin as when potassium ferricyanide is added to blood color: red-brown
5. Importance of blood sugar determination a. important in the detection of diabetes mellitus and hyperglycemia b. to diagnose toxic or inflammatory condition of the liver due to: galactosemia presence of lacic acid acidosis thymine deficiency
4. Carboxyhemoglobin and its color CO combines with heme portion of hemoglobin to form carbon monoxide hemoglobin called also carboxyhemoglobin and carbonyl hemoglobin Color: bright cherry red
c. adjust insulin dosage d. understand effects of various foods
A. Guaiac Test -bluish green color B. Benzidine Test -Bluish green color C. Hemin Test -Red brown rods
Guaiac Test and Benzidine Test bluish green color
Activity 29: Tests for Blood
Hemin Test red brown rods
3/9/2011
1. What is the chemical name and structural formula of HEMIN?
Synonyms: Ferriheme chloride, Ferriprotoporphyrin chloride 2. Describe a method on detecting blood stains on cloth.
Hemin Test -this test is quite satisfactory and the technique is simple and gives equally reliable result with fresh blood from clothes on stains as long as that it is not been exposed to the sun or high temperatures for a long period of time
IUPAC Name: chloro[3,7,12,17-tetramethyl-8,13-divinylporphyrin2,18-dipropanoato(2)]iron(III)
Activity 30: Plasma
Plasma - straw colored liquid - A watery, protein rich fluid that forms the matrix of the blood - 91.5% water and 8.5% solutes, most of which are proteins
A. Preparation of Oxalated Plasma -light yellow viscous fluid
B. Separation of Plasma Proteins and Fibrinogen 1. Plasma + distilled water + saturated ammonium sulfate sol. = light yellow solution with bubbles suspended
3. Filtrate from 2 + water + saturated ammonium sulfate sol. = cloudy light yellow solution with bubbles and white ppt.
4. Precipitate + water and perform the Biuret test = purple solution
2. Precipitate + water and perform the Biuret test = purple solution
3/9/2011
5.Filtrate from 4 contains albumin + distilled water and perform the Biuret test =purple solution 6.Heat the remaining filtrate from 4 =cloudy solution with white ppt. *Substance precipitated: albumin C. Serum 1.Serum clear yellow fluid 2. Fibrin color: red - appearance: thread like appearance 3. Millons Test red color
Questions: 1. What is the difference between plasma and serum?
Basis 1. How to be obtain? Plasma When whole blood is mixed with an anticoagulant Fluid part of blood that contains water soluble solutes 2. Components Composed of proteins, fibrinogen and clotting factors Preferred in hematology Serum When blood is allowed to clot
Fluid portion left after blood has clotted
Doesnt contain fibrinogen
3. Preference
Preferred in clinical chemistry
2. What are plasma proteins? Give the function of each. 3. What is meant by icterus index? What is its significance? Icterus index calculation of bilirubin in plasma or serum. For the determination of latent jaundice, symptom of liver disease characterized by yellow skin due to deposition of bile pigments.
10
Potrebbero piacerti anche
- Urine Analysis PracticalDocumento53 pagineUrine Analysis PracticalMubasharAbrar100% (2)
- 02 - Examination of Blood and Bone Marrow HematologyDocumento3 pagine02 - Examination of Blood and Bone Marrow Hematologyhamadadodo7Nessuna valutazione finora
- Urine ChemDocumento5 pagineUrine ChemGlenn PerezNessuna valutazione finora
- Analysis OF: UrineDocumento30 pagineAnalysis OF: UrineAebee AlcarazNessuna valutazione finora
- Al-Rasheed University Urinalysis Lab GuideDocumento24 pagineAl-Rasheed University Urinalysis Lab GuideAli RonaldoNessuna valutazione finora
- Lab Practical UrinalysisDocumento7 pagineLab Practical UrinalysisHeatherIz AwwsomeNessuna valutazione finora
- Aubf Outline EditedDocumento16 pagineAubf Outline EditedNoraine Princess TabangcoraNessuna valutazione finora
- Basic Examination of BloodDocumento6 pagineBasic Examination of BloodMadeleinePriscillaNessuna valutazione finora
- Lipids Are A Large and Diverse Group of Natural Occurring Organic Compounds That AreDocumento8 pagineLipids Are A Large and Diverse Group of Natural Occurring Organic Compounds That AregymnasrischerNessuna valutazione finora
- Non Protein CompoundsDocumento64 pagineNon Protein CompoundsAbigail Mayled LausNessuna valutazione finora
- AUBF P1 Examination Questions (1-8Documento39 pagineAUBF P1 Examination Questions (1-8Charmaine BoloNessuna valutazione finora
- Components of UrineDocumento10 pagineComponents of Urinehemanth.pharmacist100% (7)
- Emulsification LipidsDocumento23 pagineEmulsification LipidsChard RINessuna valutazione finora
- UrinalysisDocumento3 pagineUrinalysisKim MoranoNessuna valutazione finora
- Urine AnalysisDocumento41 pagineUrine AnalysisAjay SomeshwarNessuna valutazione finora
- Clinical Microscopy Lecture: Chemical Examination of UrineDocumento50 pagineClinical Microscopy Lecture: Chemical Examination of UrineThea MallariNessuna valutazione finora
- Pathological Urine ConstituentsDocumento22 paginePathological Urine Constituentsmanni1001100% (3)
- Micro Lab 1Documento16 pagineMicro Lab 1Jet MarieNessuna valutazione finora
- Clearance and GFR: Major DR Arabinda Mohan Bhattarai Lecturer (Biochemistry), NAIHSDocumento25 pagineClearance and GFR: Major DR Arabinda Mohan Bhattarai Lecturer (Biochemistry), NAIHSChandan SahNessuna valutazione finora
- Urinalysis Conclusion: Observing Urine Color, Amount, Clarity & pHDocumento2 pagineUrinalysis Conclusion: Observing Urine Color, Amount, Clarity & pHOMMONANessuna valutazione finora
- Qualitative Determination of Subcellular ComponentsDocumento30 pagineQualitative Determination of Subcellular ComponentsKarl James BañagaNessuna valutazione finora
- STAINS TABLE ArcDocumento4 pagineSTAINS TABLE ArcBenson PaglinawanNessuna valutazione finora
- Final DX ResultsDocumento9 pagineFinal DX ResultszysheaiNessuna valutazione finora
- StainingDocumento42 pagineStainingLloyd Jay LinNessuna valutazione finora
- BARFOED'S TEST Is A Chemical Test Used To Detect The Presence of Monosaccharides Which DetectsDocumento3 pagineBARFOED'S TEST Is A Chemical Test Used To Detect The Presence of Monosaccharides Which DetectsAprilNessuna valutazione finora
- FormaldehydeDocumento17 pagineFormaldehydeAe Silvestre100% (1)
- Physical Examination of UrineDocumento4 paginePhysical Examination of UrineIceNessuna valutazione finora
- BiochemLabAlviar2017 PDFDocumento26 pagineBiochemLabAlviar2017 PDFChristina Scott100% (1)
- Analysis of Physical Properties of UrineDocumento2 pagineAnalysis of Physical Properties of UrineameerabestNessuna valutazione finora
- Examination of Urine Formation and CompositionDocumento7 pagineExamination of Urine Formation and CompositionDaniel LamasonNessuna valutazione finora
- Bacte TestDocumento10 pagineBacte TestRiondalionNessuna valutazione finora
- Bacteseminar DSSMDocumento4 pagineBacteseminar DSSMPrincess AguirreNessuna valutazione finora
- Review of Aas: Pharmaceutical Biochemistry (Pha 2116)Documento41 pagineReview of Aas: Pharmaceutical Biochemistry (Pha 2116)JAKE BENZYN TENessuna valutazione finora
- Mycology 1 PrelimDocumento4 pagineMycology 1 PrelimKaye Angel VillonNessuna valutazione finora
- Group 4: Urine Screening for Metabolic DisordersDocumento41 pagineGroup 4: Urine Screening for Metabolic DisordersBrent LagartoNessuna valutazione finora
- Basic Clinical Chemistry TestsDocumento49 pagineBasic Clinical Chemistry TestsMegbaru100% (1)
- Urinalysis and Body Fluids2020Documento47 pagineUrinalysis and Body Fluids2020MONFOLA100% (1)
- Lab 3 FullDocumento17 pagineLab 3 FullAmni MohamedNessuna valutazione finora
- Aub F Urine Screening For Metabolic DisordersDocumento4 pagineAub F Urine Screening For Metabolic DisordersRomie SolacitoNessuna valutazione finora
- NPNDocumento46 pagineNPNGerald John PazNessuna valutazione finora
- Chemical Examination of UrineDocumento6 pagineChemical Examination of UrinehermanskyNessuna valutazione finora
- Lecture 10 - Urine SedimentsDocumento224 pagineLecture 10 - Urine Sedimentsdamaliso nyirongo2Nessuna valutazione finora
- Week3-Physical Examination of UrineDocumento24 pagineWeek3-Physical Examination of UrineDayledaniel SorvetoNessuna valutazione finora
- Urinalysis Cases (Revised)Documento3 pagineUrinalysis Cases (Revised)Gold NajmNessuna valutazione finora
- Subcutaneous & Systemic MycosesDocumento7 pagineSubcutaneous & Systemic MycosesDee GeeNessuna valutazione finora
- Cestodes: 1. Taenia Solium (Pork Tapeworm) & Taenia Saginata (Beef Tapeworm)Documento18 pagineCestodes: 1. Taenia Solium (Pork Tapeworm) & Taenia Saginata (Beef Tapeworm)Genevee Ryeleen DelfinNessuna valutazione finora
- Cultivation Media For BacteriaDocumento4 pagineCultivation Media For BacterialapetitefilleNessuna valutazione finora
- Perform Urine Analysis LabDocumento116 paginePerform Urine Analysis LabDr Sumant SharmaNessuna valutazione finora
- Urinalysis textbook chapters 1-8 safety quality introduction renal function physical chemical microscopic diseases screening metabolic disordersDocumento101 pagineUrinalysis textbook chapters 1-8 safety quality introduction renal function physical chemical microscopic diseases screening metabolic disordersDF DasallaNessuna valutazione finora
- Biochemical - TestsDocumento5 pagineBiochemical - TestsMohsen Haleem100% (1)
- Imvic ReactionsDocumento2 pagineImvic ReactionsAmradeepNessuna valutazione finora
- Vitamin B12 and FolateDocumento12 pagineVitamin B12 and FolateAllessandria DimaggioNessuna valutazione finora
- CLINICAL CHEMISTRY: Passbooks Study GuideDa EverandCLINICAL CHEMISTRY: Passbooks Study GuideNessuna valutazione finora
- Lecture Notes On Chemical Pathology of UrineDocumento23 pagineLecture Notes On Chemical Pathology of UrineTaylorNessuna valutazione finora
- Urine JSJ EoDocumento9 pagineUrine JSJ EokeiNessuna valutazione finora
- Biochemistry of Urine: By: Jerome S. Montano, RMTDocumento28 pagineBiochemistry of Urine: By: Jerome S. Montano, RMTAhuNessuna valutazione finora
- Urine AnalysisDocumento53 pagineUrine AnalysisMaath KhalidNessuna valutazione finora
- Urinalysis PDFDocumento56 pagineUrinalysis PDFTio AjhaNessuna valutazione finora
- URINEDocumento32 pagineURINEST12A2- Pil, StephanieNessuna valutazione finora
- بايو عملي م١Documento5 pagineبايو عملي م١tm31880388Nessuna valutazione finora
- Buffer SolutionDocumento7 pagineBuffer SolutionKirsten Hazel MejiaNessuna valutazione finora
- Urine and Blood PPT (Handout Print Form) Chua, RDocumento10 pagineUrine and Blood PPT (Handout Print Form) Chua, RKirsten Hazel Mejia100% (1)
- CSI ID Format and Guidelines Summer 2011Documento3 pagineCSI ID Format and Guidelines Summer 2011Kirsten Hazel MejiaNessuna valutazione finora
- Exp 3 ParasitDocumento5 pagineExp 3 ParasitKirsten Hazel MejiaNessuna valutazione finora
- Glomerular Disorders 2Documento3 pagineGlomerular Disorders 2Kirsten Hazel MejiaNessuna valutazione finora
- Allow Me To Begin With A Short Story About PepengDocumento1 paginaAllow Me To Begin With A Short Story About PepengKirsten Hazel MejiaNessuna valutazione finora
- Epithelial TissuesDocumento5 pagineEpithelial TissuesKirsten Hazel MejiaNessuna valutazione finora
- Bacteriology - Chapter One The Bacterial Cell: Prokaryotes and EukaryotesDocumento139 pagineBacteriology - Chapter One The Bacterial Cell: Prokaryotes and EukaryotesKirsten Hazel MejiaNessuna valutazione finora
- The Legacy of PAMET PresidentsDocumento2 pagineThe Legacy of PAMET PresidentsKirsten Hazel Mejia100% (1)
- Book of EstherDocumento4 pagineBook of EstherKirsten Hazel MejiaNessuna valutazione finora
- Biochemistry Course OverviewDocumento5 pagineBiochemistry Course OverviewKirsten Hazel MejiaNessuna valutazione finora
- 24 Hour Urine CollectionDocumento3 pagine24 Hour Urine CollectionFatima Neshreen SarahadilNessuna valutazione finora
- Caffeine boosts endurance sports performanceDocumento17 pagineCaffeine boosts endurance sports performanceMaks MiljanNessuna valutazione finora
- Nursing Management of Chronic Hypertension with Superimposed PreeclampsiaDocumento40 pagineNursing Management of Chronic Hypertension with Superimposed PreeclampsiaRaidis Pangilinan100% (2)
- Early Care and Development (Ecd) Checklist Gross Motor Domain Pre PostDocumento3 pagineEarly Care and Development (Ecd) Checklist Gross Motor Domain Pre PostJuicy May LansangNessuna valutazione finora
- Navidas-Case StudyDocumento5 pagineNavidas-Case StudyFran LanNessuna valutazione finora
- Top lab tests and codesDocumento18 pagineTop lab tests and codesZyralyn PanganibanNessuna valutazione finora
- PHYSICAL EXAMINATION OF URINE NotesDocumento3 paginePHYSICAL EXAMINATION OF URINE NotesAlarice CnNessuna valutazione finora
- Kidney Stone Owner's Manual v2Documento12 pagineKidney Stone Owner's Manual v2Martin HaynesNessuna valutazione finora
- Science-Investigatory-Project-Andres FINALLLDocumento27 pagineScience-Investigatory-Project-Andres FINALLLElynn AlianganNessuna valutazione finora
- Evaluation of Alcoholic and Aqueous Extracts of Nicandra Physalodes Leaves For Diuretic ActivityDocumento4 pagineEvaluation of Alcoholic and Aqueous Extracts of Nicandra Physalodes Leaves For Diuretic ActivityThota VijaykumarNessuna valutazione finora
- Bag Technique and Urine Testing GuideDocumento25 pagineBag Technique and Urine Testing GuideJairene Dave Martinez Cambalon100% (1)
- BIO202L+Lab+14+ The Urinary SystemDocumento9 pagineBIO202L+Lab+14+ The Urinary Systemmyra Thiong'oNessuna valutazione finora
- AlkalineDocumento86 pagineAlkalineYildiz Aytac100% (1)
- Krauses Food and The Nutrition Care Process 13th Edition Mahan Test BankDocumento8 pagineKrauses Food and The Nutrition Care Process 13th Edition Mahan Test Bankajarinfecternl3vs100% (28)
- NCP Karl Jacon M. FerolinoDocumento4 pagineNCP Karl Jacon M. FerolinoWhyng CabugayanNessuna valutazione finora
- CULTURE URINE: The Standard Procedure For A Valid Sample ProceduresDocumento3 pagineCULTURE URINE: The Standard Procedure For A Valid Sample ProceduresFranca CerquaNessuna valutazione finora
- Proper urine collection maximizes lab test diagnosisDocumento26 pagineProper urine collection maximizes lab test diagnosisClaryse EroyNessuna valutazione finora
- NCM - 109 RLE Worksheet No. 4 Common Diagnostics - Laboratory and Nursing Procedures Related To Renal and Reproductive DisordersDocumento10 pagineNCM - 109 RLE Worksheet No. 4 Common Diagnostics - Laboratory and Nursing Procedures Related To Renal and Reproductive DisorderskdfhjfhfNessuna valutazione finora
- DLP - ScienceDocumento7 pagineDLP - ScienceJUDE ABBUNessuna valutazione finora
- Pathophysiology of Ureteral CalculiDocumento14 paginePathophysiology of Ureteral CalculiEdmel Pamplona DuquesaNessuna valutazione finora
- 1 Urinary StonesDocumento11 pagine1 Urinary StonesMohamed Al-zichrawyNessuna valutazione finora
- Class 4 - Circulatory and Excretory System Questions and AnswersDocumento4 pagineClass 4 - Circulatory and Excretory System Questions and Answersanil guptaNessuna valutazione finora
- Multistix Reagent StripDocumento3 pagineMultistix Reagent StripFrancis TorresNessuna valutazione finora
- UntitledDocumento2 pagineUntitledVictorious AtnapNessuna valutazione finora
- Toxicity and Toxicokinetics of Metformin in RatsDocumento26 pagineToxicity and Toxicokinetics of Metformin in RatsPooja ReddyNessuna valutazione finora
- 2nd Lecture Normal Phsiological Changes of Aging Final1.Ppt40-41Documento85 pagine2nd Lecture Normal Phsiological Changes of Aging Final1.Ppt40-41halayehiahNessuna valutazione finora
- Sidra Internship ReportDocumento40 pagineSidra Internship Reporthafeez ahmedNessuna valutazione finora
- Sample Brochure 2Documento1 paginaSample Brochure 2Reinn Dionisio100% (1)
- Renal Diseases PathophysiologyDocumento6 pagineRenal Diseases PathophysiologyBilly Gayados100% (1)
- How Increase of Bilirubin in Blood Impacts Cooking Likeness?Documento3 pagineHow Increase of Bilirubin in Blood Impacts Cooking Likeness?IIJSR JournalNessuna valutazione finora