Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Experiment 4 510
Caricato da
syafiqa123Descrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Experiment 4 510
Caricato da
syafiqa123Copyright:
Formati disponibili
EXPERIMENT 4 ANALYSIS OF HYDROCARBONS IN COMMON FUELS BY SOLID-PHASE MICROEXTRACTION (SPME) AND GAS CHROMATOGRAPHY MASS SPECTROMETRY
Introduction Gasoline, diesel and kerosene are all created from crude oil by a variety of refining and distillation processes. Each product is produced by the combination of multiple individual hydrocarbon compounds all of which have slightly different vaporization and boiling temperatures. Gasoline is the combination of many lower boiling range compounds while the middle boiling range compounds are used in differing proportions to create kerosene and diesel. The profile of hydrocarbons in oil may hence be used to characterize the oil. This enables the identification of the candidate source of oil spill cases.
Solid-phase microextraction (SPME) parameters were examined on water contaminated with hydrocarbons including benzene and alkylbenzenes, n-alkanes, and polycyclic aromatic hydrocarbons (PAHs). Sample preparation is an essential step in analysis, greatly influencing the reliability and accuracy of analysis. It is a rapid, inexpensive and solventless technique for the isolation of organic compounds from gaseous and liquid sample. It is based on the enrichment of the analytes on a polymer or adsorbent coated fused silica fiber by exposing the fiber directly or to do its headspace and thermal desorption of the analytes in the GC injector. In this experiment, the components of hydrocarbons in common fuels are identified using SPME-GC-MS
SPME Apparatus The SPME apparatus consist of a fiber holder and a fiber assembly, the latter containing a 1 to 2 cm long retractable SPME fiber. The SPME fiber is a thin fused-silica optical fiber (polydimethylsiloxane, PDMS) Factor that effect SPME performance Coating material The most important features determining the analytical performance of SPME is the type and thickness of the coating material. The selectivity of extraction depends on the type of fiber. In general, polar fibers are used for polar analytes and non-polar fibers for non-polar analytes as with the conventional GC stationary phases. The use of a thicker fiber requires a longer extraction time but the recoveries are generally higher. The time of extraction is independent of the concentration of analyte in the sample and the relative number of molecules extracted at a distinct time is also independent of the concentration of analyte. Usually the thinnest acceptable film is employed to reduce extraction times. Extraction Procedure The coated fibre is immersed directly in the sample or the headspace of the sample, where the analytes are concentrated. After equilibrium has been reached (froma few minutes to several hours depending on the properties of the analytes measured) or after a defined time the fiber is withdrawn and transferred either to a GC injection port. The extraction efficiency and the time required to reach equilibrium can be influenced by several factors that include extraction temperature, sample agitation, volatility of analytes and type, thickness of fiber coating.
Sample Accelerates: Unleaded petrol, diesel, paint thinner and kerosene.
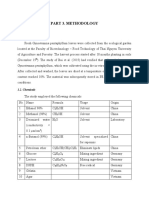
Apparatus: SPME holder with 100 m polydimethylsiloxane (PDMS) fibre. Instrument 1. Gas chromatograph (Agilent Technologies 5890 Series II) equipped with HP 5971A mass selective detector and a 30 m x 250 m x 0.25 m HP 5-MS capillary column. 2. Glass vial with septum.
Analytical Procedure 1. Instrument set-up Injector temperature Detector temperature Carrier gas flow rate Column temperature : 250 C : 300 C : 30 mL/s : 60 C to 170 C/min
2. SPME Procedure i. The fiber condition in the GC injection port at 250 C for at least 10 minutes to removes contaminants. ii. 5 mL of sample was adding approximately in a glass vial and the vial was place on a hot plate. The sample was heat up to 50 C. The sample was agitated by using the magnetic stirrer. iii. iv. v. The SPME fiber was exposed to the headspace of the vial for 20 minutes Withdraw the fiber into the needle, pull out from the vial and immediately inject into the GC-MS with a desorption time 80 second. Using the mass spectra library, identify the major compounds in each sample using the mass spectra library.
Discussion: In this experiment, the sample preparation used solid phase microextraction (SPME).SPME reduces the time necessary for sample preparation, decreases purchases and disposal costs of solvents and can improve detection limits. That is why the SPME was choose in this analysis. From this experiment, there are four chromatograms obtain which are kerosene, petrol, diesel and unknown. The method used was SPME where it used fiber to absorb the sample. Needle with fiber was put into the sample by head space method. The sample was volatile and thermally stable. So it does not need to dip it into the sample. The sample was volatile when it heated and the fiber absorbs the sample volatile and keeps it in the fiber. The first chromatogram obtain was diesel sample. There are eight main components in the sample. However only four components with the high quality were taken and the structure were taken from the library of the mass spectrometer. The components taken were heptadecane with 97 quality, hexadecane with 98 quality, tetradecane with 97 quality, and p-xylene with 95 qualities. The second sample was injected was kerosene. There were a lot of component in this sample which were 31 component found in the chromatogram. The biggest quality for the component to read in the library were undecane with 94 quality, benzene, 1-methyl-3-prophyl with 90 quality, decane with the 94 quality, and tetradecane with a quality of 98. The third sample injected was petrol there are nine component found in this sample. Unfortunately only three components were taken to detect the structure. First structure with the 95 quality was benzene, 1, 2, 3,-trimethyl. Second component was p-xylene with 95 qualities. The third component was toluene with 91 qualities. The fourth sample injected was unknown. There were 14
component detected in this sample. From this component, the result was compared with the other three standards. It was found that in the unknown contain the mixture of kerosene and diesel. This was due to the component of p-xylene, o-xylene, and pxylene in the unknown. This component existent is similar to the component detect in the diesel compound. Also component of dodecane, tetradecane and tridecane shows of diesel compound in the unknown. To show the existent of the kerosene compound in the unknown was the detection of compound of benzene 1, 2, 3-trimethyl, benzene 1, 3, 5-trimethyl, benzene 1, 2, 4-trimethyl, which is very similar with kerosene standard. Another compound was found were component of benzene 1-ethyl-2-methyl, benzene 1-ethyl-3-methyl, benzene 1-ethyl-3- methyl. To prove that the unknown has a kerosene compound was the detection of component naphthalene 1-methyl in both unknown and kerosene sample. Conclusion: The hydrolysis of hydrocarbon in common fuels by solid phase microextraction (SPME) and gas chromatography mass spectrometry (GC-MS) was determined and the unknown was a mixture of kerosene and diesel compound. Reference: i. Skoog.D.A. West.D.M. Holler.F.J. Crouch.S.R.(2004). Fundamental of Analytical Chemistry. 8th ed. Thomson Brooks/Cole. Pg 947, 954.
Potrebbero piacerti anche
- Anise Oil as a Precursor for PMA SynthesisDocumento12 pagineAnise Oil as a Precursor for PMA Synthesisaml_zgz8233% (3)
- Xuanbo Gao, Shukui Zhu, Wanfeng Zhang, Donghao Li, Wei Dai, Sheng HeDocumento10 pagineXuanbo Gao, Shukui Zhu, Wanfeng Zhang, Donghao Li, Wei Dai, Sheng HeKartika AnggraeniNessuna valutazione finora
- GC-MS Experiment Identifies HydrocarbonsDocumento8 pagineGC-MS Experiment Identifies HydrocarbonsSyazwani MalekNessuna valutazione finora
- Analysis of Hydrocarbons in Common Fuels by Solid-Phase Microextraction (Spme) and Gas Chromatography - Mass Spectrometry (GC-MS)Documento6 pagineAnalysis of Hydrocarbons in Common Fuels by Solid-Phase Microextraction (Spme) and Gas Chromatography - Mass Spectrometry (GC-MS)Amirul Azhar100% (11)
- Wax DeterminationDocumento12 pagineWax Determinationdrojas70Nessuna valutazione finora
- Lab Report SPMEDocumento8 pagineLab Report SPMEAyish MataNessuna valutazione finora
- Theory and Technology of Multiscale Dispersed Particle Gel for In-Depth Profile ControlDa EverandTheory and Technology of Multiscale Dispersed Particle Gel for In-Depth Profile ControlNessuna valutazione finora
- PNS en 14103 - Fame PDFDocumento13 paginePNS en 14103 - Fame PDFWynona BasilioNessuna valutazione finora
- EXP5SPMEDocumento13 pagineEXP5SPMEungu_sakura50% (2)
- Lab Report - Determination of Accelerants in Fire Debris Using Solid Phase MicroextractionDocumento6 pagineLab Report - Determination of Accelerants in Fire Debris Using Solid Phase MicroextractionNur Atiqah AhmadNessuna valutazione finora
- Phenols in FuelDocumento12 paginePhenols in Fuelvzimak2355Nessuna valutazione finora
- Experment 4 Analysis of Hydrocarbonsin Common Fuels Using Solid-Phase Microextraction (SPME) and Gas Chromatography-Mass Spectrometry (GC-MS)Documento6 pagineExperment 4 Analysis of Hydrocarbonsin Common Fuels Using Solid-Phase Microextraction (SPME) and Gas Chromatography-Mass Spectrometry (GC-MS)NUR IZZATI OTHMAN BASRINessuna valutazione finora
- Headspace and SDMEDocumento2 pagineHeadspace and SDMEAlex IsacNessuna valutazione finora
- Cuantificacion Diesel HPLCDocumento8 pagineCuantificacion Diesel HPLCJose Antonio Martinez VillalbaNessuna valutazione finora
- Oxidaçao LipidicaDocumento7 pagineOxidaçao LipidicaLaura MascarinNessuna valutazione finora
- Gcms ReportDocumento20 pagineGcms Reportapi-355003548Nessuna valutazione finora
- 0018AN - Performance of Method 8270 Using Hydrogen Carrier GasDocumento4 pagine0018AN - Performance of Method 8270 Using Hydrogen Carrier GasAuxiliar SySNessuna valutazione finora
- Parameters Affecting The Accelerated Solvent Extraction of Polymeric SamplesDocumento6 pagineParameters Affecting The Accelerated Solvent Extraction of Polymeric SamplesdeepongkarNessuna valutazione finora
- 2005 - Journal of Chromatography BDocumento6 pagine2005 - Journal of Chromatography BDina MansourNessuna valutazione finora
- Journal of Chromatography A:, Mahmoud Ebrahimi, Mohammad-Saeid HosseiniDocumento7 pagineJournal of Chromatography A:, Mahmoud Ebrahimi, Mohammad-Saeid HosseinijubuhlerNessuna valutazione finora
- Rapid Analysis of Liquid Polyester Resins UsingDocumento9 pagineRapid Analysis of Liquid Polyester Resins UsingnikythekNessuna valutazione finora
- PCBs Determination in Transformer Oil by SPE and GCDocumento10 paginePCBs Determination in Transformer Oil by SPE and GCAditya Febrian MasriNessuna valutazione finora
- D2638Documento3 pagineD2638rimi7alNessuna valutazione finora
- Testing & Quality Control Lab Report (8) : Title: To Check Colorfastness To Dry-CleaningDocumento6 pagineTesting & Quality Control Lab Report (8) : Title: To Check Colorfastness To Dry-CleaningAsad Jamil RanaNessuna valutazione finora
- Atomic Absortion Spectroscop yDocumento15 pagineAtomic Absortion Spectroscop yVân Khánh NguyễnNessuna valutazione finora
- Eurl-Fv Multires Idue Method Using Mini-Luke Followed by Gc-Qqq-Ms/Ms For Fruits and VegetablesDocumento11 pagineEurl-Fv Multires Idue Method Using Mini-Luke Followed by Gc-Qqq-Ms/Ms For Fruits and VegetablesTu TranNessuna valutazione finora
- The Effects of Temperature and Pressure On The Performance of Carboxen/PDMS Fibres During Solid Phase Microextraction (SPME) of Headspace Volatiles From Cooked and Raw Turkey BreastDocumento9 pagineThe Effects of Temperature and Pressure On The Performance of Carboxen/PDMS Fibres During Solid Phase Microextraction (SPME) of Headspace Volatiles From Cooked and Raw Turkey BreastРусланNessuna valutazione finora
- Determinación COVs en aguas por CG-MS HeadspaceDocumento6 pagineDeterminación COVs en aguas por CG-MS HeadspaceCristian CarrascoNessuna valutazione finora
- Analysis of Methanol and Ethanol in Virgin Olive OilDocumento6 pagineAnalysis of Methanol and Ethanol in Virgin Olive OilHabibatus LatifahNessuna valutazione finora
- VOC Analysis of Gasoline Samples by GCDocumento10 pagineVOC Analysis of Gasoline Samples by GCammarnizamuddinNessuna valutazione finora
- Experiment 9 - Analysis of Fibre Dyes by HPLCDocumento5 pagineExperiment 9 - Analysis of Fibre Dyes by HPLCNur Atiqah AhmadNessuna valutazione finora
- Hargis 2013Documento4 pagineHargis 2013HungChiHoNessuna valutazione finora
- Closing the Mass Balance on Fluorine on Papers and TextilesDocumento19 pagineClosing the Mass Balance on Fluorine on Papers and TextilesCTNessuna valutazione finora
- Painel ENQA v2Documento1 paginaPainel ENQA v2Jocinei DogniniNessuna valutazione finora
- GC MS ReportDocumento10 pagineGC MS ReportWahyuniAntariNessuna valutazione finora
- Gas Chromatography (GC), Optimization of Flow Rate and Column TemperatureDocumento6 pagineGas Chromatography (GC), Optimization of Flow Rate and Column TemperatureAmirul Azhar86% (14)
- 27 873 PDFDocumento6 pagine27 873 PDFMerlando Dany SNessuna valutazione finora
- 17 Ac19Documento9 pagine17 Ac19Dana StoinNessuna valutazione finora
- additif antioxydantDocumento8 pagineadditif antioxydantIsaac MabongaNessuna valutazione finora
- MethodologyDocumento17 pagineMethodologyChung TrinhNessuna valutazione finora
- Article1379954058 - Yasin Et AlDocumento11 pagineArticle1379954058 - Yasin Et AlMuhammad Imran KhanNessuna valutazione finora
- EPA Method - 604 - 1984 PDFDocumento23 pagineEPA Method - 604 - 1984 PDFpatobohrNessuna valutazione finora
- CPSC CH E1002 08 - 2Documento9 pagineCPSC CH E1002 08 - 2ericakwokNessuna valutazione finora
- Eduardo Pereira, César Cáceres, Fabiola Rivera, Bernabé Rivas, Pedro SáezDocumento8 pagineEduardo Pereira, César Cáceres, Fabiola Rivera, Bernabé Rivas, Pedro SáezNadia Putri UtamiNessuna valutazione finora
- CPSC CH E1002 08 - 1Documento6 pagineCPSC CH E1002 08 - 1ericakwokNessuna valutazione finora
- Propionico AireDocumento11 paginePropionico AireLaboratorio HelgueroNessuna valutazione finora
- Edible Veg OilsDocumento12 pagineEdible Veg OilsmarianaivanovaprofNessuna valutazione finora
- Report 4 GCDocumento26 pagineReport 4 GCNurhafizah Abd JabarNessuna valutazione finora
- Exempt PaintDocumento6 pagineExempt Paintkmdollar89Nessuna valutazione finora
- Thermodynamic and kinetic parameters of polyester dyeing with Disperse Blue 56Documento14 pagineThermodynamic and kinetic parameters of polyester dyeing with Disperse Blue 56Ami SaNessuna valutazione finora
- Analysis of Benzo (A) PyreneDocumento14 pagineAnalysis of Benzo (A) PyreneECRDNessuna valutazione finora
- 9 - New Approach To Analysis of Formaldehyde in Water Soluble PolymersDocumento4 pagine9 - New Approach To Analysis of Formaldehyde in Water Soluble Polymerslinhpic99Nessuna valutazione finora
- Poly-å-Caprolactam: 1. ProcedureDocumento3 paginePoly-å-Caprolactam: 1. ProcedureMathaneshan RajagopalNessuna valutazione finora
- Identification of Odor Components of AgarwoodDocumento5 pagineIdentification of Odor Components of AgarwoodRiza RinjaniNessuna valutazione finora
- Optical Fiber Sensor for Biodiesel Quality AnalysisDocumento4 pagineOptical Fiber Sensor for Biodiesel Quality AnalysisLuluk MalikNessuna valutazione finora
- Environmental Pollutants—Selected Analytical Methods: Scope 6Da EverandEnvironmental Pollutants—Selected Analytical Methods: Scope 6Nessuna valutazione finora
- Monitoring Toxic Gases in the Atmosphere for Hygiene and Pollution Control: Pergamon International Library of Science, Technology, Engineering and Social StudiesDa EverandMonitoring Toxic Gases in the Atmosphere for Hygiene and Pollution Control: Pergamon International Library of Science, Technology, Engineering and Social StudiesValutazione: 3.5 su 5 stelle3.5/5 (17)