Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
1995 AL Chemistry Paper 1 Marking Scheme
Caricato da
api-3738841100%(1)Il 100% ha trovato utile questo documento (1 voto)
2K visualizzazioni21 pagineCopyright
© Attribution Non-Commercial (BY-NC)
Formati disponibili
PDF o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Attribution Non-Commercial (BY-NC)
Formati disponibili
Scarica in formato PDF o leggi online su Scribd
100%(1)Il 100% ha trovato utile questo documento (1 voto)
2K visualizzazioni21 pagine1995 AL Chemistry Paper 1 Marking Scheme
Caricato da
api-3738841Copyright:
Attribution Non-Commercial (BY-NC)
Formati disponibili
Scarica in formato PDF o leggi online su Scribd
Sei sulla pagina 1di 21
URSLEGSBY FOR TEACHERS’ USE ONLY
Qa
Page tata
SECTION A
‘Answer ALL questions in this Section. Write your answers in the spaces provided
L (2) @_—_ The first four successive ionization energies of an element A are 578, 1817, 2746 and
10813 1d mot" respectively. To which group in the Periodic Table does 4 belong ?
Group If / ITIA / 3 / 3a 1
Gi) The relative atomic mass of bromine is 79.90. It has two isotopes with mass numbers
79 and 81
Calculate the relative abundance of each isotope.
Assuming "ar 1 “ar = x: (1- x)
19x + (1 - «)81 = 79.90
x= 0.55
Abundance of er = 558
“ae = 458
G masks)
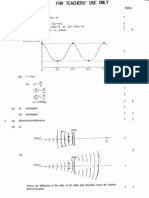
(©) i) What can you deduce from the fact that the spectral lines in the atomic emission
spectrum of hydrogen are nor equally spaced ?
‘The energy differences between electron shells in a
hydrogen atom are not the sane (converge)
Gi) Inthe atomic emission spectrum of hydrogen, the convergence limit for the Lyman series
‘occurs at 3.275 x 10% Hz. Calculate the ionization energy of hydrogen, in kJ mol”
(Planck constant, h = 6.626 x 10™ 5s; Avogadeo constant, L,= 6.023 x 10 mol!)
eB. = ho
= 6.526 X 10™ x 3.275 x 10 x 6,023 x 108 1
= 1.307 x 106 3 mol
(2307 te mot) (i 305-1310 KS ne)
(4 mark for numerical answer; 4 mark for unit) 1
@ marks)
9S-AL-CHEM 1-2
FABRAXRAEB === FOR TEACHERS? USE ONLY
{2 Account for the fact that the carbon-oxygen bond lengths in CO, CO, and CO," are 0.113, 0.116
and 0.129 nm respectively.
co C86. / 36
4
co, orczo f CHF CEO. 4
co,” It is a resonance hybrid of the following:
é &
‘or ator 4
eae tig Seer cath eevee 5
6 ay
(a) The iodination of propanone is catalysed by hydrogen ions. The overall equation is
CH,COCH(aq) + Iyaq) > CH,COCH,K(ag) + HIkaq)
Using four mixtures B, C, D and E, the progress of the reaction was followed by colorimetric,
measurement. The cesults are tabulated below,
‘Composition by volume of mixture / cm
Initial rate
Mixture | propanone | water | 100MHCI| 0.05M i, in KI | /moldm?s* |.
B 10.0 60.0 10.0 20.0 4.96 & 10%
© 10.0 50.0 10.0 30.0 3.04 x 10%
D 35.0 65.0 10.0 20.0 2.45 x 10"
E 10.0 65.0 5.0 20.0 2.47 x10"
() Determine the effects of the changes in concentration of each of the reactants (iodine and
propanone) and the catalyst (hydrochloric acid) oa the resction rate, Write the rate
expression for the reaction.
h , use 8, C + rate independent of (12)
propanone, use 8, D : rate a (propanone} box
a ,use B, Bo: rate a [#7] -2
rate =k (H*) (CH,COCH,]
(ii) For mixture B, calculate the rate constant for the reaction at the temperature of the
‘experiment,
(Density of CH,COCH, = 0.789 g cm”)
si 100_x 0.78
ate = 4.96 x 104 = k (0.2) (ORDER) 1
59.078 x 4.96 x 10"
ke = 3665 X10 a ge
0.1 x 100 x 0.789 peter :
4 marks)
(4 mark for numerical anawer; 4 mark for unit)
95-AL-CHEM 3
ARAM SE FOR TEACHERS?’ USE ONLY
Qa
Tase uta
(2) The reaction between ethanoic acid and ethanol can be represented by the following equation:
CH,COOH() + CHOH® * CH,COOCHAD + H,0()
12.01 g of ethanoie acid are treated with 4.61 g of ethanol in the presence of a catalyst. When
the reaction reaches equilibrium at 298 K, 5.04 g of ethanoic acid are found to have reacted.
(Name a suitable catalyst for this reaction in the forward direction,
concentrated sulphuric(VI) acid/hydrochieric acid/
hydrogen enloride gas 2
(Calculate the equilibrium constant, K, , for the reaction at 298 K.
acid + “alechol = eater + water
initial no. 12.01 461 7 2
of moles 60.052 5.088
= 0.2 = OL
no. of moles 12:01 = 3:08 4.1 _ 0.084 0.084 0.084
at eqn. 60.082
= 0.116 = 0,016 axe
0.089"
K. * 3.80 (no unit )
eT ONE OO OT ety) CAceept 3-80 ~3-70)
(y for answer; 4 for unity 1
i) What additional mass of ethanol would be required in order to use up a further 0.60 g
of ethanoie acid ?
0.6 g of ethanoic acid = 0.01 mole
acid + alcohol- * ester + water
no. of moles
at eqn. 0.106 * 0.094 0.09445
9.094
3-80 = “Q106%
woe = 0.0219 mol, 4s
need to add 0.016 mol of ethariol = 0.74 g 4
(0-732 00.7423
Gv) Would the addition of more of the same catalyst affect the value of Ke? Explain.
No 4
catalyst can affect only the rate of (forward and backward) 4
reaction.
(7 marks)
9S-AL-CHEM 4
Potrebbero piacerti anche
- 1993 AL Chemistry Paper I Marking SchemeDocumento7 pagine1993 AL Chemistry Paper I Marking Schemeapi-373884150% (2)
- 1996 AL Chemistry Marking SchemeDocumento34 pagine1996 AL Chemistry Marking Schemeapi-373884175% (4)
- 1990 AL Chemistry Paper I Marking SchemeDocumento11 pagine1990 AL Chemistry Paper I Marking Schemeapi-373884160% (5)
- 1997 AL Chemistry Paper 2 Marking SchemeDocumento12 pagine1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- 1994 AL Chemistry Paper I Marking SchemeDocumento10 pagine1994 AL Chemistry Paper I Marking Schemeapi-3738841100% (1)
- 2000 AL Chemistry Paper I Marking SchemeDocumento20 pagine2000 AL Chemistry Paper I Marking Schemeapi-3738841Nessuna valutazione finora
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocumento36 pagine1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- 2003 AL Chemistry Paper 1 Marking SchemeDocumento16 pagine2003 AL Chemistry Paper 1 Marking Schemeapi-3738841100% (1)
- 2000 AL Chemistry Paper II Marking SchemeDocumento15 pagine2000 AL Chemistry Paper II Marking Schemeapi-3738841Nessuna valutazione finora
- 1997 AL Chemistry Paper I Marking SchemeDocumento22 pagine1997 AL Chemistry Paper I Marking Schemeapi-3738841Nessuna valutazione finora
- 1999 AL Chemistry Marking SchemeDocumento34 pagine1999 AL Chemistry Marking Schemeapi-373884125% (4)
- 2001 AL Chemistry Paper II Marking SchemeDocumento16 pagine2001 AL Chemistry Paper II Marking Schemeapi-373884163% (8)
- AL 1995 Physics Marking Scheme IIBDocumento7 pagineAL 1995 Physics Marking Scheme IIBapi-3738841100% (1)
- AL 1991 Physics Marking SchemeDocumento22 pagineAL 1991 Physics Marking Schemeapi-373884150% (2)
- 1999 AL Chemistry Marking SchemeDocumento34 pagine1999 AL Chemistry Marking Schemeapi-373884125% (4)
- 2002 AL Chemistry Paper 1+2 Marking SchemeDocumento36 pagine2002 AL Chemistry Paper 1+2 Marking Schemeapi-373884138% (8)
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocumento36 pagine1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- AL 1990 Physics Marking Scheme IIADocumento13 pagineAL 1990 Physics Marking Scheme IIAapi-373884167% (3)
- 1997 AL Chemistry Paper 2 Marking SchemeDocumento12 pagine1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- AL 1991 Physics Marking Scheme IIADocumento12 pagineAL 1991 Physics Marking Scheme IIAapi-37388410% (3)
- AL 1992 Physics Marking SchemeDocumento22 pagineAL 1992 Physics Marking Schemeapi-373884186% (7)
- AL 1993 Physics Marking SchemeDocumento16 pagineAL 1993 Physics Marking Schemeapi-3738841100% (3)
- AL 1994 Physics Marking Scheme IADocumento14 pagineAL 1994 Physics Marking Scheme IAapi-3738841100% (2)
- AL 2000 Physics Marking SchemeDocumento40 pagineAL 2000 Physics Marking Schemeapi-373884180% (10)
- AL 1994 Physics Marking Scheme IIBDocumento14 pagineAL 1994 Physics Marking Scheme IIBapi-3738841Nessuna valutazione finora
- AL 1997 Physics Marking SchemeDocumento15 pagineAL 1997 Physics Marking Schemeapi-373884167% (3)
- AL 1996 Physics Marking SchemeDocumento14 pagineAL 1996 Physics Marking Schemeapi-3738841100% (4)
- AL 2001 Physics Marking SchemeDocumento16 pagineAL 2001 Physics Marking Schemeapi-373884125% (8)
- AL 1999 Physics Marking SchemeDocumento15 pagineAL 1999 Physics Marking Schemeapi-373884150% (2)
- AL 1998 Physics Marking SchemeDocumento15 pagineAL 1998 Physics Marking Schemeapi-373884175% (8)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)