Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Dicom Dryview6850
Caricato da
Jose QuiscaTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Dicom Dryview6850
Caricato da
Jose QuiscaCopyright:
Formati disponibili
DRYVIEW 6850 Laser Imager
Quality Control Manual for Mammography
Published by:
Carestream Health, Inc.
Rochester, NY 14608
This document is based upon the following template:
NEMA Standards Publication XR 23-2006
Quality Control Manual Template for Manufacturers of
Hardcopy Output Devices
Labeled for Final Interpretation in Full-field Digital Mammography
Published by:
National Electrical Manufacturers Association
1300 North 17th Street, Suite 1752
Rosslyn, Virginia 22209
www.nema.org
NOTICE AND DISCLAIMER
from the NEMA Template
The information in this publication was considered technically sound by the consensus of persons
engaged in the development and approval of the document at the time it was developed.
Consensus does not necessarily mean that there is unanimous agreement among every person
participating in the development of this document.
The National Electrical Manufacturers Association (NEMA) standards and guideline publications, of
which the document contained herein is one, are developed through a voluntary consensus
standards development process. This process brings together volunteers and/or seeks out the
views of persons who have an interest in the topic covered by this publication. While NEMA
administers the process and establishes rules to promote fairness in the development of
consensus, it does not write the document and it does not independently test, evaluate, or verify
the accuracy or completeness of any information or the soundness of any judgments contained in
its standards and guideline publications.
NEMA disclaims liability for any personal injury, property, or other damages of any nature
whatsoever, whether special, indirect, consequential, or compensatory, directly or indirectly
resulting from the publication, use of, application, or reliance on this document. NEMA disclaims
and makes no guaranty or warranty, express or implied, as to the accuracy or completeness of any
information published herein, and disclaims and makes no warranty that the information in this
document will fulfill any of your particular purposes or needs. NEMA does not undertake to
guarantee the performance of any individual manufacturer or sellers products or services by virtue
of this standard or guide.
In publishing and making this document available, NEMA is not undertaking to render professional
or other services for or on behalf of any person or entity, nor is NEMA undertaking to perform any
duty owed by any person or entity to someone else. Anyone using this document should rely on
his or her own independent judgment or, as appropriate, seek the advice of a competent
professional in determining the exercise of reasonable care in any given circumstances.
Information and other standards on the topic covered by this publication may be available from
other sources, which the user may wish to consult for additional views or information not covered
by this publication.
NEMA has no power, nor does it undertake to police or enforce compliance with the contents of
this document. NEMA does not certify, test, or inspect products, designs, or installations for safety
or health purposes. Any certification or other statement of compliance with any health or safety
related information in this document shall not be attributable to NEMA and is solely the
responsibility of the certifier or maker of the statement.
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page i
CONTENTS
Foreword.iii
Section 1 OVERVIEW OF THE QUALITY CONTROL MANUAL............................................................... 1
1.1
Scope of the Document ............................................................................................................. 1
1.2
Regulatory Considerations ........................................................................................................ 1
1.3
Structure of the Document......................................................................................................... 1
1.4
Elements of a QC/Constancy Test ............................................................................................ 2
1.5
The Need for Calibration ........................................................................................................... 2
1.6
Test Summary ........................................................................................................................... 2
Section 2 QC TESTS FOR THE RADIOLOGIC TECHNOLOGIST ............................................................ 4
2.1
2.2
2.3
2.4
2.5
Density Constancy Test............................................................................................................. 4
2.1.1 Objective....................................................................................................................... 4
2.1.2 Establishing Operating Levels...................................................................................... 4
2.1.3 Performing the QC/Constancy Test ........................................................................... 10
Fixer Retention Test (N/A)....................................................................................................... 11
Low-contrast Visibility Test ...................................................................................................... 11
2.3.1 Objective..................................................................................................................... 11
2.3.2 Establishing Operating Levels.................................................................................... 11
2.3.3 Performing the QC/Constancy Test ........................................................................... 13
Spatial Resolution Test............................................................................................................ 13
2.4.1 Objective..................................................................................................................... 13
2.4.2 Establishing Operating Levels.................................................................................... 13
2.4.3 Performing the QC/Constancy Test ........................................................................... 14
(Darkroom) Fog Test (N/A)...................................................................................................... 15
2.6
Artifact Test.............................................................................................................................. 15
2.6.1 Objective..................................................................................................................... 15
2.6.2 Establishing Operating Levels.................................................................................... 15
2.6.3 Performing the QC/Constancy Test ........................................................................... 17
Section 3 QC TESTS FOR THE MEDICAL PHYSICIST........................................................................... 18
3.1
Grayscale Response Function Test ........................................................................................ 18
3.1.1 Objective..................................................................................................................... 18
3.1.2 Establishing Operating Levels.................................................................................... 18
3.1.3 Performing the QC/Constancy Test ........................................................................... 20
3.2
Geometry Test ......................................................................................................................... 20
3.2.1 Objective..................................................................................................................... 20
3.2.2 Establishing Operating Levels.................................................................................... 21
3.2.3 Performing the QC/Constancy Test ........................................................................... 23
3.3
Phantom Image Quality Test ................................................................................................... 23
3.3.1 Objective..................................................................................................................... 23
3.3.2 Establishing Operating Levels.................................................................................... 23
3.3.3 Performing the QC/Constancy Test ........................................................................... 23
Section 4 GUIDANCE ................................................................................................................................ 25
4.1
QC Test Images ...................................................................................................................... 25
4.2
Density Constancy Test (2.1) .................................................................................................. 26
4.3
Fixer Retention Test (2.2) (N/A) .............................................................................................. 26
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page ii
4.4
Low-Contrast Visibility Test (2.3)............................................................................................. 26
4.5
Spatial Resolution Test (2.4) ................................................................................................... 26
4.6
(Darkroom) Fog Test (2.5) (N/A) ............................................................................................. 26
4.7
Artifact Test (2.6) ..................................................................................................................... 26
4.8
Grayscale Response Function Test (3.1)................................................................................ 27
4.9
Geometry Test (3.2) ................................................................................................................ 27
4.10
Phantom Image Quality Test (3.3) .......................................................................................... 27
4.11
Mammography Equipment Evaluation (MEE) ......................................................................... 27
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page iii
Foreword
Quality Control (QC) is important in any imaging system, but it is especially important in mammography.
When full-field digital mammography (FFDM) systems were first introduced, all components (e.g., image
receptor, acquisition workstation, diagnostic workstation/monitor, hardcopy output device) were provided
or qualified for use by the image receptor manufacturer (IRM). The IRM also provided a comprehensive
QC plan to enable mammography facilities to meet their responsibilities under the Mammography Quality
Standards Act1 (MQSA). Subsequently, FDA approved manufacturers other than the IRMs to market
hardcopy and softcopy displays for FFDM images. This has made system QC more difficult since, under
MQSA regulations, the facility is required to follow a quality assurance program substantially the same as
the one recommended by the IRM. However, the QC plan of the IRM may not adequately address the
needs of components developed by other manufacturers.
The increasing heterogeneity of FFDM systems has created a desire to delegate the responsibility for
developing QC procedures for the individual system components to the manufacturers of those
components. This manual provides such procedures for the 6850 Laser Imager, a hardcopy output
device that receives images from an FFDM system, and reproduces them on film for final interpretation.
The procedures in this manual cover two main performance dimensions: grayscale fidelity and
spatial/geometric fidelity. The grayscale dimension addresses the ability of the hardcopy device to
achieve and maintain constancy in its tone reproduction characteristics relative to established operating
levels. The spatial dimension measures the ability of the device to achieve and maintain the appropriate
level of spatial resolution, geometric constancy, and freedom from artifacts.
The United States Mammography Quality Standards Act of 1992, as amended by the Mammography
Quality Standards Reauthorization Acts of 1998 and 2004 (MQSRA).
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
< This page is intentionally left blank. >
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
Page iv
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 1
Section 1
OVERVIEW OF THE QUALITY CONTROL MANUAL
1.1
SCOPE OF THE DOCUMENT
This document defines the minimum set of quality control (QC) tests to be applied to the DRYVIEW 6850
Laser Imaging System, a hardcopy output device that receives images from an FFDM system, and
reproduces them on film for final interpretation. It should be considered as one element of the
mammography facilitys Quality Assurance Plan.
The tests in this manual generally focus on device constancy, that is, the ability of the hardcopy device to
maintain its expected imaging performance over time. They do not address system quality control, for
example, the ability of the various system components (image receptor, acquisition workstation,
diagnostic workstations, hardcopy output device) to communicate properly with each other, and to use
the communicated information correctly. Such system QC activities are assumed to have occurred
during initial system installation, initial system acceptance, or as part of a Mammography Equipment
Evaluation (see 4.11).
1.2
REGULATORY CONSIDERATIONS
Facilities subject to the provisions of the Mammography Quality Standards Act (MQSA) that use image
receptors other than screen/film must follow a quality assurance program substantially the same as
the one recommended by the image receptor manufacturer (21 CFR 900.12(e)(6)). It remains the
responsibility of the facility to determine whether or not this QC plan is substantially the same as the one
recommended by the image receptor manufacturer for the FFDM system(s) in use at that facility.
1.3
STRUCTURE OF THE DOCUMENT
This QC manual is categorized according to whether the test must be performed by a:
Radiologic technologist (Section 2), or a
Medical physicist (Section 3).
Within each category, QC tests are arranged according to test frequency, starting with daily/weekly tests,
and moving on to quarterly, semi-annual, and annual tests. Each QC test is further split into two parts:
Establishing operating levels
The operating levels are the values against which subsequent, periodic QC test results for the
device must be compared. While they change relatively infrequently, certain events can trigger
the (re)establishment of operating levels. Examples of such events are significant changes to
hardware or software, changes in output media (e.g., film) type, or media batch/lot number, and
anything that would require the performance of a Mammography Equipment Evaluation. Media
type is defined on the labeling provided by its manufacturer, and relates to properties such as
base, tint, tone, density range, or other differentiating characteristics.
Performing the QC/Constancy test
This test determines if, and by how much, the hardcopy output device is deviating from the
currently valid operating levels.
Finally, a Guidance section (Section 4) is included that summarizes Carestream Health, Inc.s current
thinking on performing the various QC tests.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
1.4
Page 2
ELEMENTS OF A QC/CONSTANCY TEST
Each QC/Constancy test contains a set of standard elements consistent with the requirements and intent
of the MQSA:
Objective of the test
Frequency of testing
Equipment required for the test (beyond the calibrated hardcopy output device)
Procedures and conditions to perform the test
Action limits, or tolerances, for the test results
Use of test results (see 21 CFR 900.12(e)(8))
If the test results are outside the action limits, the necessary user actions are specified. For the
tests in this manual, this action is generally to stop further printing of mammograms with the
failing device until the source of the problem has been identified and corrected. For only one
test (2.2, Fixer Retention, which is not applicable to the 6850 Laser Imager), does MQSA
currently allow the corrective action to be taken within 30 days of the test.
Equipment lists, procedures, action limits, and actions based on state-of-the-art devices or current
practice are provided for each test.
1.5
THE NEED FOR CALIBRATION
All test procedures in this manual assume a properly calibrated hardcopy output device. The calibration
procedure for the 6850 Laser Imager is described in the online Help or the Users Guide for the 6850
Laser Imager. The user shall ensure that the hardcopy device is calibrated any time operating levels are
established, and any time QC/Constancy testing is done.
Several tests also require a calibrated densitometer.
A commercial, external densitometer must be used for these tests. Its calibration shall
conform to the frequency and procedure specified by the densitometer manufacturer. Further,
the user shall ensure that the densitometer is calibrated at the time of use, and maintain
records documenting its calibration history.
1.6
TEST SUMMARY
Table 1-1 summarizes the tests covered in this QC manual, their minimum frequencies, the person
responsible for each test, and the sections of this manual in which details about each test can be found.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 3
Table 1-1
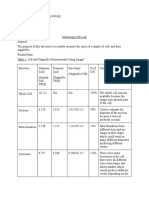
QUALITY CONTROL TESTS WITH TIMING REQUIREMENTS AND RESPONSIBILITIES
Responsibility
When to (re)establish
operating levels
When to perform
QC test
Section
Grayscale
Radiologic
Technologist
See 2.1.2.1 for
triggering events
Weekly
(see 2.1.3.1)
and during MEE
2.1
Fixer Retention*
Grayscale
Not Applicable
to 6850 Laser
Imager
Not Applicable to
6850 Laser Imager
2.2
Low-contrast Visibility
Grayscale
Radiologic
Technologist
See 2.3.2.1 for
triggering events
Semi-annually
and during MEE
2.3
Spatial Resolution
Spatial/Geometric
Radiologic
Technologist
See 2.4.2.1 for
triggering events
Semi-annually
and during MEE
2.4
Not Applicable to
6850 Laser Imager
2.5
Test Name
Density Constancy
Test Type
Not Applicable
to 6850 Laser
Imager
(Darkroom) Fog*
Grayscale
Artifact
Spatial/Geometric
Grayscale Response Function
Not Applicable to 6850 Laser
Imager
MQSA defines levels:
21 CFR 900.12(e)(3)(i)
Not Applicable to 6850 Laser
Imager
MQSA defines levels:
21 CFR 900.12(e)(4)(i)
Radiologic
Technologist
See 2.6.2.1 for
triggering events
Annually
and during MEE
2.6
Grayscale
Medical
Physicist
See 3.1.2.1 for
triggering events
Annually
and during MEE
3.1
Geometry
Spatial/Geometric
Medical
Physicist
See 3.2.2.1 for
triggering events
Annually
and during MEE
3.2
Phantom Image Quality
System
Medical
Physicist
Mammography Equipment
Evaluation (MEE)
Annually
and during MEE
3.3
*This test is required only under certain conditions. See the listed Section and the Guidance (Section 4) for more information.
NOTE All of the above QC tests, with the exception of Fixer Retention and (Darkroom) Fog, must be done as part of any Mammography Equipment Evaluation (MEE) involving
the hardcopy device. An MEE must be done, for example, when new equipment is installed, or existing equipment undergoes changes or repairs that might affect imaging
performance. See the Guidance (4.11) for more information.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 4
Section 2
QC TESTS FOR THE RADIOLOGIC TECHNOLOGIST
2.1
DENSITY CONSTANCY TEST
2.1.1
Objective
To ensure that the hardcopy output device reproduces consistently a representative set of densities
within its output density range.
2.1.2
Establishing Operating Levels
2.1.2.1
Triggering Events
Equipment installation/acceptance, major changes to the hardcopy devices exposure and processing
hardware components, software, image processing parameters or other events that would necessitate a
Mammography Equipment Evaluation (MEE). Operating levels must also be reestablished if the
densitometer used in these tests is replaced, repaired or changed in any way other than normal userperformed calibration using a valid (not expired) density step tablet.
2.1.2.2
Equipment Required
For Procedure 1 below, digital test image containing a density step wedge that produces at least the
following nominal output optical densities. (See Table 1 below for the definitions of the four nominal
output optical densities.) For the 6850 Laser Imager, these four densities are measured using four
selected steps from an internally stored 21-step Density QC test pattern. Specifically, either the Density
QC, 8X10, Density QC, 10x12 or Density QC, 11x14 should be selected for printing from the imagers
user interface, depending on the film size that will be used in these QC tests. Each of these test patterns
includes two columns (step wedges), each having 21 density steps, as shown next:
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 5
Example of a QC Test Pattern (Density QC, 8X10)
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 6
As a convenience to the user, writing space is provided in boxes on the right side of these films
where the user can record the step numbers that are being used and the densitometer
measurements of those steps. On initial prints that are made to establish operating levels, only
the four final reference density values and their step numbers (as determined in section 2.1.2.3
below) are recorded in the Reference boxes. On the films subsequently printed for density
constancy testing, the previously established reference density values (and their step numbers)
are reentered in the Reference boxes, the current films measured values (from the same step
numbers that were previously used) are entered in the Measured boxes, and calculated
measured reference values are entered in the Difference boxes.
Here are the four nominal output optical densities:
Table 1. Nominal Output Optical Densities
-Minimum density (Dmin): must correspond to the digital driving level that produces the
minimum density on the specified output medium under
normal operating conditions In a Density QC step wedge,
the step labeled 1 is always the step to be used for
measuring Dmin.
-Low Density:
density closest to, but not less than 0.45
-Mid-Density:
density closest to, but not less than 1.20
-High Density:
density closest to, but not less than 2.20
Calibrated densitometer. The step wedge that is closest to the film edge can be read, in its
entirety, with an automatic strip-reading densitometer such as the X-RITE Model 391
Densitometer. Alternatively, the step wedge that is closest to the middle of the film can be read,
one step at a time, with a densitometer such as the X-RITE Model 301.
Quality Control charts to track the density variation of each of the above steps over time.
Measured densities are recorded for the operating levels, and variations from these are charted
using the Laser Imager Quality Control Chart. User remarks and actions are recorded in the
Laser Imager Quality Control Chart Log. These forms are illustrated below.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 7
Laser Imager Quality Control Chart
Imager____________Film Lot_________________ Month/Year_____ Densitometer__________ Wedge_________
Month
Date
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31
+ 0.03
Dmin=
Step# 1
- 0.03
+ 0.07
Low D=
Step#__
- 0.07
+ 0.15
Mid D=
Step#__
- 0.15
+ 0.15
High D=
Step#__
- 0.15
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 8
Laser Imager Quality Control Chart Log
Remarks
2.1.2.3
Date
Action
Procedure
1) Print the digital test image which is matched to the film size being used (Density QC, 8X10 for 8X10,
Density QC, 10x12 for 10X12, or Density QC, 11x14 for 11X14 film) twice and measure the following
density steps in each printed image:
Dmin
Low Density
Mid-Density
High Density
To determine and record these four measured densities, and their associated step numbers, the following
procedure is used:
1. To ensure that the imager is calibrated, first print an imager calibration sheet using film from the
cartridge that will be used in the subsequent density constancy tests. This will cause the imager
to automatically update its built-in calibration.
2. Next, make two prints of the Density QC test image corresponding to this same film size, from the
same cartridge.
3. Starting with a new, blank Laser Imager Quality Control Chart, fill in the top line with:
a. The Laser Imager Serial #
b. The film lot from the cartridge being used
c. The month and year of the current date
d. The Model # and Serial # of the calibrated densitometer which will be used throughout
these tests
e. The wedge choice (center or edge) which will be used throughout these tests
4. Starting with a new, blank worksheet such as the following, record all 21 densities from the
wedge being used on each of the two films just printed and enter those data into the second and
third columns of the worksheet.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 9
Worksheet for Determining Operating Levels Densities
Step #
Measured
Density,
QC Test Print
#1
Measured
Density,
QC Test Print
#2
Measured
Density,
Average
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
Step Usage
Dmin
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 10
5. From the second and third columns of data, calculate the average measured density of each of
the 21 steps and enter these averages into the fourth column.
6. Take the average density for Step 1 and write it on the Laser Imager Quality Control Chart
immediately to the right of Dmin=. This average density value will be used as the Dmin
reference density value for all subsequent Dmin (Step 1) constancy measurements, until
operating levels are reestablished and a new chart is started.
7. To determine which step to use as the Low Density step, scan, from the top through the data in
the second and third columns, stopping at the first step in which the measured densities in both
of the first two columns are at least 0.45. On the worksheet, write Low Density in the Step
Usage column on the row for this step number. Take the average density at that step and write it
on the Laser Imager Quality Control Chart immediately to the right of Low D=. Also write the
step number on the next line of the chart, after Step#. This step number and average density
value will be used as the Low Density step number and reference density value for all
subsequent Low Density constancy measurements, until operating levels are reestablished and a
new chart is started.
8. To determine which step to use as the Mid Density step, scan, from the top through the data in
the second and third columns, stopping at the first step in which the measured densities in both
of the first two columns are at least 1.20. On the worksheet, write Mid Density in the Step
Usage column on the row for this step number. Take the average density at that step and write it
on the Laser Imager Quality Control Chart immediately to the right of Mid D=. Also write the
step number on the next line of the chart, after Step#. This step number and average density
value will be used as the Mid Density step number and reference density value for all subsequent
Mid Density constancy measurements, until operating levels are reestablished and a new chart is
started.
9. To determine which step to use as the High Density step, scan, from the top through the data in
the second and third columns, stopping at the first step in which the measured densities in both
of the first two columns are at least 2.20. On the worksheet, write High Density in the Step
Usage column on the row for this step number. Take the average density at that step and write it
on the Laser Imager Quality Control Chart immediately to the right of High D=. Also write the
step number on the next line of the chart, after Step#. This step number and average density
value will be used as the High Density step number and reference density value for all
subsequent High Density constancy measurements, until operating levels are reestablished and
a new chart is started.
2.1.2.4
Action Limits
If the imagers printed QC wedges do not contain densities ranging from 0.45 through 2.20, the imager
requires a service call, and no further printing of mammograms may be done until the imager is repaired.
2.1.3
Performing the QC/Constancy Test
2.1.3.1
Frequency
Because the 6850 Laser Imager is a hardcopy device that utilizes a dry development process and selfcalibration, this test needs only to be done on a weekly basis.
2.1.3.2
Equipment Required
Digital Density QC Test Pattern test image printed under the same conditions (e.g., look-up
tables, interpolation, maximum density) used to establish operating levels
Calibrated densitometer (preferably, the same densitometer used to establish operating levels)
Quality Control chart containing the current operating levels established in 2.1.2
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
2.1.3.3
Page 11
Procedure
Print the test image once and measure the relevant density steps (Dmin, low, mid-, and high densities).
Record the step numbers and their corresponding densities on the QC chart.
2.1.3.4
Action Limits
Dmin must be within:
Low density must be within:
Mid-density must be within:
High density must be within:
2.1.3.5
0.03 of the operating level
0.07 of the operating level
0.15 of the operating level
0.15 of the operating level
Use of Test Results
If the device fails the test, further printing of mammograms with the device must stop until the source of
the problem is identified and corrected. As a first step towards correcting the problem, initiate a print of
an imager calibration sheet, then print the Density QC Test Pattern again and check its four densities
against the existing reference baseline values. If the four new measured-reference values are all within
the action limits defined above, mammograms may continue to be printed, and these newest measuredreference values may be charted, but the recalibration of the imager should be noted in the log. If any of
the four new measured-reference values lie outside the above action limits, initiate a completely new
charting cycle using the procedure of section 2.1.2.3, and note the need to do this in the logs of both the
previous and the new charts. If the test failure is likely due to use of a new batch/lot number from the
same media type, note this in the logs, after performing the procedure of section 2.1.2.3.
2.2
FIXER RETENTION TEST (N/A)
This test, which is included in the NEMA template, is not applicable to DRYVIEW Laser Imagers since
they do not utilize wet chemical processing with a chemical fixing agent.
2.3
LOW-CONTRAST VISIBILITY TEST
2.3.1
Objective
To assess the ability of the hardcopy output device to reproduce low-contrast structures across its density
range.
2.3.2
Establishing Operating Levels
2.3.2.1
Triggering Events
Equipment installation/acceptance, major changes to the hardcopy devices hardware or software, other
events that would necessitate a Mammography Equipment Evaluation (MEE).
2.3.2.2 Equipment Required
Digital test image containing low-contrast structures embedded in known surrounding densities (all
structures with known gray levels). For the 6850 Laser Imager, the test image Multipurpose QC <film
size> Test Image should be selected from the user interface, for true-size printing on whatever film size
is in current use for these QC tests. The Multipurpose QC Test Image is shown next.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Multipurpose QC <film size>
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
Page 12
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
2.3.2.3
Page 13
Procedure
From the 6850 user interface, make one print of the Multipurpose QC.
Place the printed film on a light box that is normally used for viewing mammograms, and ensure that
viewing conditions (such as ambient light and shuttering) are as they normally would be for diagnostic
interpretation of mammograms.
In the central part of the test pattern, which is designed to approximately simulate a mammography
accreditation phantom image (but without x-ray capture system noise), count the numbers of visible
(simulated) fibrils, speck groups and masses and write those numbers in the spaces provided on the film.
2.3.2.4
Action Limits
All six fibrils, all five speck groups, and all five masses in the simulated phantom must be visible. If they
are not all visible, as a first step towards correcting the problem, initiate a print of an imager calibration
sheet, then re-print the Multipurpose QC and re-check the visibilities of all the simulated fibrils, speck
groups and masses. If some are still not visible, a service call must be placed.
2.3.3
Performing the QC/Constancy Test
2.3.3.1
Frequency
Semi-annually.
2.3.3.2
Equipment Required
Digital Multipurpose QC Test Image printed under the same conditions (e.g., look-up tables,
interpolation, maximum density) used to produce hardcopy reference image.
2.3.3.3
Procedure
Print the test image and evaluate the visibility of low-contrast structures.
2.3.3.4
Action Limits
All six fibrils, all five speck groups, and all five masses in the simulated phantom must be visible.
2.3.3.5
Use of Test Results
If the device fails the test, further printing of mammograms with the device must stop until the source of
the problem is identified and corrected. As a first step towards correcting the problem, initiate a print of
an imager calibration sheet, then re-print the Multipurpose QC Image and re-check the visibilities of all
the simulated fibrils, speck groups and masses. If some are still not visible, a service call must be placed.
2.4
SPATIAL RESOLUTION TEST
2.4.1
Objective
To assess the ability of the hardcopy output device to reproduce fine spatial details.
2.4.2
Establishing Operating Levels
2.4.2.1
Triggering Events
Equipment installation/acceptance, major changes to the hardcopy devices hardware or software, other
events that would necessitate a Mammography Equipment Evaluation (MEE).
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
2.4.2.2
Page 14
Equipment Required
Digital test image containing spatial-resolution targets/test patterns. For the 6850 Laser Imager, the test
image Multipurpose QC <film size>, as described previously, should be selected from the user interface,
for true-size printing on whatever film size is in current use for these QC tests.
2.4.2.3
Procedure
From the 6850 user interface, make one print of the Multipurpose QC. (Alternatively, a film that was just
printed for the Low-Contrast Visibility Test previously described may also be used for this test.)
Place the printed film on a light box that is normally used by radiologists for viewing mammograms, and
ensure that viewing conditions (such as ambient light and shuttering) are as they normally would be for
diagnostic interpretation of mammograms.
Using a loupe or other magnifier with 5X 10X magnification, look near the top of the image, in the
horizontal strip containing vertical bar patterns at various spatial frequencies. See if all, including the
finest bars (labeled 12.8 Line pairs per millimeter), can be seen (i.e., there is not just a uniform shade of
gray there).
Similarly, using the same loupe or other magnifier, look near the right side of the image, in the vertical
strip containing horizontal bar patterns at various spatial frequencies. See if all, including the finest bars
(labeled 12.8 Line pairs per millimeter), can be seen (i.e., there is not just a uniform shade of gray
there).
On the film, answer the question Are all line pairs per millimeter bars visible? by checking either the
Yes box or the No box on the film. A Yes answer here means that all were visible, including the
finest (12.8 line pairs per mm), on both the horizontal strip and the vertical strip.
2.4.2.4
Action Limits
The answer to the question Are all line pairs per millimeter bars visible? must be Yes. If the answer is
No, as a first step towards correcting the problem, initiate a print of an imager calibration sheet, then reprint the Multipurpose QC and re-check, with magnification as defined above, the visibilities of all the bar
patterns. If some are still not visible, try printing the Multipurpose QC using film from a different film
cartridge. If this does not solve the problem, a service call must be placed.
2.4.3
Performing the QC/Constancy Test
2.4.3.1
Frequency
Semi-annually.
2.4.3.2
Equipment Required
Digital test image containing spatial-resolution targets/test patterns. For the 6850 Laser Imager, the test
image Multipurpose QC <film size>, as described previously, should be selected from the user interface,
for true-size printing on whatever film size is in current use for these QC tests.
2.4.3.3
Procedure
Print the digital test image and check the visibility of the specified spatial-resolution targets/structures.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
2.4.3.4
Page 15
Action Limits
Using a loupe or other magnifier with 5X 10X magnification, the viewers answer to the question Are all
line pairs per millimeter bars visible? must be Yes.
2.4.3.5
Use of Test Results
If the device fails the test, further printing of mammograms with the device must stop until the source of
the problem is identified and corrected. If the printer fails this test, then, as a first step towards correcting
the problem, initiate a print of an imager calibration sheet. Then re-print the Multipurpose QC and recheck, with magnification as defined above, the visibilities of all the bar patterns. If some are still not
visible, try printing the Multipurpose QC using film from a different film cartridge. If this does not solve the
problem, a service call must be placed.
2.5
(DARKROOM) FOG TEST (N/A)
This test, which is included in the NEMA template, is not applicable to the 6850 Laser Imager since its
cartridge design prevents unintended exposure of media during normal daylight handling.
2.6
ARTIFACT TEST
2.6.1
Objective
To assess the ability of the hardcopy output device to produce images that are free from clinically
significant artifacts (i.e., artifacts that mimic or obscure clinical information).
2.6.2
Establishing Operating Levels
2.6.2.1
Triggering Events
Equipment installation/acceptance, major changes to the hardcopy devices hardware or software, other
events that would necessitate a Mammography Equipment Evaluation (MEE). This test may also be
triggered by an observation of a possible artifact on a printed clinical film.
2.6.2.2
Equipment Required
Digital test image(s) containing large, mid-density, flat-field areas that aid in detecting relevant artifacts.
For the 6850 Laser Imager, the Carestream Health, Inc. test image TG18 QC should be selected from
the user interface. The TG18 QC is shown next.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
TG18 QC
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
Page 16
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
2.6.2.3
Page 17
Procedure
Print the test image and evaluate for any artifacts seen in the hardcopy output that mimic or obscure
clinical information.
2.6.2.4
Action Limits
There must be no artifacts that mimic or obscure clinical information. If these, or any other artifact which
may mimic or obscure clinical information, are seen, a different film cartridge should be placed in the
imager and the test repeated, to see if the artifact is associated with a particular cartridge of film. If the
artifact still occurs, the imager may require cleaning or other maintenance. In this case, a service call
must be placed and clinical printing suspended until the problem is corrected. If the artifact disappeared
when the original cartridge was replaced, the original cartridge must no longer be used for clinical
printing.
2.6.3
Performing the QC/Constancy Test
2.6.3.1
Frequency
Annually.
2.6.3.2
Equipment Required
Digital test images printed under the same conditions (e.g., look-up tables, interpolation, maximum
density) used to produce hardcopy reference images.
2.6.3.3
Procedure
Print the two test images and evaluate for any artifacts seen in the hardcopy output that mimic or obscure
clinical information.
2.6.3.4
Action Limits
There must be no artifacts that mimic or obscure clinical information.
2.6.3.5
Use of Test Results
If the device fails the test, further printing of mammograms with the device must stop until the source of
the problem is identified and corrected. If any artifact is seen which may mimic or obscure clinical
information, a different film cartridge should be placed in the imager and the test repeated, to see if the
artifact is associated with a particular cartridge of film. If the artifact still occurs, the imager may require
cleaning or other maintenance. In this case, a service call must be placed and clinical printing suspended
until the problem is corrected. If the artifact disappeared when the original cartridge was replaced, the
original cartridge must no longer be used for clinical printing.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 18
Section 3
QC TESTS FOR THE MEDICAL PHYSICIST
The following QC tests shall be done by a medical physicist or by an individual under the direct
supervision of a medical physicist, typically in conjunction with the annual mammography facility survey.
These tests also form the basis of the Mammography Equipment Evaluation (see 4.11).
3.1
GRAYSCALE RESPONSE FUNCTION TEST
3.1.1
Objective
To ensure that the grayscale response of the hardcopy output device conforms to the expected grayscale
response.
3.1.2
Establishing Operating Levels
3.1.2.1
Triggering Events
Equipment installation/acceptance, major changes to the hardcopy devices hardware or software,
changes in media (e.g., film) type, other events that would necessitate a Mammography Equipment
Evaluation (MEE).
3.1.2.2
Equipment Required
Digital test image containing a sufficient number of density steps with known gray levels.
Depending on the film size in use, one of the three Density QC images, previously described in
section 2.1, may be printed for use with this test. Specifically, if printing on 8x10 film, print the
Density QC, 8x10 test image; if printing on 10x12 film, print the Density QC, 10x12 test image;
if printing on 11x14 film, print the Density QC, 11x14 test image. In all cases, the central 21-step
wedge of the test image is used for this test. The steps in this wedge contain pixel values ranging
from 0 (0% = black) through 4095 (100% = white), with a uniform increment of 5% in pixel value
per step.
Calibrated X-Rite Model 301 densitometer or other densitometer with equivalent accuracy and
visual spectral response may be used for this test. Before this test is performed, the
densitometer must be calibrated with a current and valid X-Rite Part No. 301-27 density
reference plaque.
3.1.2.3
Procedure
The following procedure describes how the accuracy of printed densities, throughout the printable
grayscale range, is tested, using a prescribed look-up table, in conjunction with prescribed printing and
film parameters.
First, to ensure that the imager is calibrated, print an imager calibration sheet using film from the cartridge
that will be used in the subsequent test pattern print. This will cause the imager to automatically update
its built-in calibration.
Next, the appropriate Density QC is printed from the user interface. For this test, print parameters are
automatically set to the following:
Look-up table: Transfer Function Table VER693C0, contrast 6
Dmax Setting: 3.60
Print Format: 1-up (single image, magnified to fill most of the film)
Interpolation: Replicate
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 19
The Automatic Image Quality Control (AIQC) feature of the 6850 Laser Imager automatically expands the
chosen lookup table to span the full available density range from the current films Dmin to the requested
Dmax. Therefore, the expected densities on the printed film will depend to some extent on the Dmin of
the film in the cartridge that is currently being used. Film Dmin can be determined by measuring the
density of the brightest step (Step 1) of the printed wedge. For the print parameters being used here, and
a range of Dmin possibilities, the expected printed densities for all 21 steps are given, along with their
tolerances, in the following table.
Target Densities and Tolerances for the QC Wedge, Printed as Specified, for Various Dmins
Step
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
Dmin
0.17
0.17
Dmin
0.18
0.18
Dmin
0.19
0.19
Dmin
0.20
0.20
Dmin
0.21
0.21
Dmin
0.22
0.22
Dmin
0.23
0.23
Dmin
0.24
0.24
Dmin
0.25
0.25
Dmin
0.26
0.26
Dmin
0.27
0.27
0.24
0.1
0.30
0.1
0.37
0.1
0.44
0.1
0.52
0.1
0.60
0.1
0.69
0.1
0.78
0.1
0.88
0.1
0.99
0.1
1.11
0.1
1.24
0.15
1.38
0.15
1.55
0.15
1.73
0.15
1.94
0.15
2.21
0.15
2.54
0.15
2.95
0.15
3.60
0.25
0.25
0.1
0.31
0.1
0.38
0.1
0.45
0.1
0.53
0.1
0.61
0.1
0.70
0.1
0.79
0.1
0.89
0.1
1.00
0.1
1.12
0.11
1.24
0.15
1.39
0.15
1.56
0.15
1.74
0.15
1.95
0.15
2.22
0.15
2.55
0.15
2.95
0.15
3.60
0.25
0.26
0.1
0.32
0.1
0.39
0.1
0.46
0.1
0.54
0.1
0.62
0.1
0.71
0.1
0.80
0.1
0.90
0.1
1.01
0.1
1.13
0.11
1.25
0.15
1.40
0.15
1.56
0.15
1.74
0.15
1.95
0.15
2.22
0.15
2.55
0.15
2.95
0.15
3.60
0.25
0.27
0.1
0.33
0.1
0.40
0.1
0.47
0.1
0.55
0.1
0.63
0.1
0.72
0.1
0.81
0.1
0.91
0.1
1.02
0.1
1.13
0.11
1.26
0.15
1.41
0.15
1.57
0.15
1.75
0.15
1.96
0.15
2.23
0.15
2.56
0.15
2.96
0.17
3.60
0.25
0.28
0.1
0.34
0.1
0.41
0.1
0.48
0.1
0.56
0.1
0.64
0.1
0.73
0.1
0.82
0.1
0.92
0.1
1.03
0.1
1.14
0.12
1.27
0.15
1.42
0.15
1.58
0.15
1.76
0.15
1.97
0.15
2.24
0.15
2.56
0.15
2.96
0.17
3.60
0.25
0.29
0.1
0.35
0.1
0.42
0.1
0.49
0.1
0.57
0.1
0.65
0.1
0.73
0.1
0.83
0.1
0.93
0.1
1.04
0.1
1.15
0.12
1.28
0.15
1.43
0.15
1.59
0.15
1.77
0.15
1.98
0.15
2.24
0.15
2.57
0.15
2.97
0.19
3.60
0.25
0.30
0.1
0.36
0.1
0.43
0.1
0.50
0.1
0.58
0.1
0.66
0.1
0.74
0.1
0.84
0.1
0.94
0.1
1.05
0.1
1.16
0.13
1.29
0.15
1.44
0.15
1.60
0.15
1.78
0.15
1.99
0.15
2.25
0.15
2.58
0.15
2.97
0.19
3.60
0.25
0.31
0.1
0.37
0.1
0.44
0.1
0.51
0.1
0.59
0.1
0.67
0.1
0.75
0.1
0.85
0.1
0.95
0.1
1.06
0.1
1.17
0.13
1.30
0.15
1.44
0.15
1.61
0.15
1.78
0.15
1.99
0.15
2.26
0.15
2.58
0.15
2.97
0.19
3.60
0.25
0.32
0.1
0.38
0.1
0.45
0.1
0.52
0.1
0.60
0.1
0.68
0.1
0.76
0.1
0.85
0.1
0.96
0.1
1.06
0.1
1.18
0.14
1.31
0.15
1.45
0.15
1.61
0.15
1.79
0.15
2.00
0.15
2.27
0.15
2.59
0.15
2.98
0.21
3.60
0.25
0.33
0.1
0.39
0.1
0.46
0.1
0.53
0.1
0.61
0.1
0.69
0.1
0.77
0.1
0.86
0.1
0.96
0.1
1.07
0.1
1.19
0.14
1.32
0.15
1.46
0.15
1.62
0.15
1.80
0.15
2.01
0.15
2.27
0.15
2.59
0.15
2.98
0.21
3.60
0.25
0.34
0.1
0.40
0.1
0.47
0.1
0.54
0.1
0.62
0.1
0.70
0.1
0.78
0.1
0.87
0.1
0.97
0.1
1.08
0.1
1.20
0.14
1.33
0.15
1.47
0.15
1.63
0.15
1.81
0.15
2.02
0.15
2.28
0.15
2.60
0.15
2.99
0.23
3.60
0.25
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 20
The expected step densities and tolerances on a given print are found by taking the column from the
above table that corresponds to the current films measured Dmin. (Since this column is freely chosen to
match (to within 0.01) the current films measured Dmin, there is no difference between the target and
measured densities at Step 1. Thus no tolerance is indicated for the Step 1 density.)
3.1.2.4
Action Limits
Taking the target densities and tolerances from the column above that corresponds to the Dmin (Step 1
density) of the current film, the measured and target densities must agree to within the defined tolerances
for all 21 steps of the wedge. If they do not, this procedure (3.1.2.3) should be repeated. If acceptable
results are still not obtained, the current film cartridge should be replaced with a new one and the entire
procedure (3.1.2.3) repeated one more time. If results are still unsatisfactory, a service call must be
placed to correct the problem before these tests, or any clinical image printing, may be resumed.
3.1.3
Performing the QC/Constancy
3.1.3.1
Frequency
Annually.
3.1.3.2
3.1.3.3
Equipment Required
The same digital test image used in section 3.1.2.2, to be printed under the same conditions
(e.g., look-up tables, interpolation, maximum density).
Calibrated densitometer (preferably, the same densitometer used to establish operating levels).
Procedure
First, to ensure that the imager is calibrated, print an imager calibration sheet using film from the cartridge
that will be used in the subsequent test pattern print. This will cause the imager to automatically update
its built-in calibration.
Next, the appropriate Density QC is printed, as previously described in section 3.1.2.3.
As in section 3.1.2.3, measure the densities of the 21 steps, and compare them with the column from the
Target Densities and Tolerances table above that corresponds to the Dmin (Step 1 density value) of the
newly printed film. (The new films Dmin need not be the same as that of the film used in section 3.1.2.3.)
3.1.3.4
Action Limits
The new measured densities must agree with the target densities to within the defined tolerances for all
21 steps of the newly printed wedge.
3.1.3.5
Use of Test Results
If the device fails the test, further printing of mammograms with the device must stop until the source of
the problem is identified and corrected. If any out-of tolerance results were obtained, procedure 3.1.3.3
should be repeated. If acceptable results are still not obtained, the current film cartridge should be
replaced with a new one and the entire procedure, starting with section 3.1.2.3, should be repeated. If
results are still unsatisfactory, a service call must be placed to correct the problem before these tests, or
any clinical image printing, may be resumed.
3.2
GEOMETRY TEST
3.2.1
Objective
To ensure that the hardcopy output device preserves linearity and distance in both horizontal and vertical
dimensions.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
3.2.2
Establishing Operating Levels
3.2.2.1
Triggering Events
Page 21
Equipment installation/acceptance, major changes to the hardcopy devices hardware or software, other
events that would necessitate a Mammography Equipment Evaluation (MEE).
3.2.2.2 Equipment Required
Digital test image containing multiple horizontal and vertical lines having a specified spatial
relationship (e.g., fixed distance) to each other. Specifically, for the 6850 Laser Imager, the
TG18-QC, described previously, is printed.
A precision flat steel ruler, marked in mm increments, longer than the longest dimension of the
films used in this test.
QC Chart. The Geometry Test QC Chart shown below is used to record the results of this test.
When new operating levels are established using this procedure, a new Geometry Test QC Chart
is begun, and the new operating levels are recorded on the top line of the new chart.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 22
Geometry Test QC Chart
Date
Horizontal Distance, mm
Vertical Distance, mm
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
Straightness of Lines
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
3.2.2.3
Page 23
Procedure
Print the test image and measure horizontal and vertical reference distances within the image. Record
these on a QC chart. Examine the printed lines.
3.2.2.4
Action Limits
Lines must be straight and undistorted. If it appears that the vertical lines on the film are not straight and
undistorted, measure the horizontal distance between them at three locations: near the top edge of the
film, across the middle of the film, and near the bottom edge of the film. If the difference between the
longest and the shortest of these distances exceeds 2% of the average distance, a service call must be
placed and the problem resolved before clinical images can be printed. Similarly, if it appears that the
horizontal lines on the film are not straight and undistorted, measure the vertical distance between them
at three locations: near the left edge of the film, near the vertical centerline of the film, and near the right
edge of the film. If the difference between the longest and the shortest of these distances exceeds 2% of
the average distance, a service call must be placed and the problem resolved before clinical images can
be printed.
3.2.3
Performing the QC/Constancy Test
3.2.3.1
Frequency
Annually.
3.2.3.2
Equipment Required
Digital test image printed under the same conditions (e.g., look-up tables, interpolation, maximum
density) used to produce hardcopy reference image
QC Chart containing the current operating levels established in 3.2.2
3.2.3.3
Procedure
Print the test image and measure the horizontal and vertical reference distances within the image.
Record these on the QC chart, and compare with the reference values. Examine the printed lines.
3.2.3.4
Action Limits
Measured distances must not deviate by more than 1% from the reference distances. Lines must be
straight and undistorted.
3.2.3.5
Use of Test Results
If the device fails the test, further printing of mammograms with the device must stop until the source of
the problem is identified and corrected. A service call is required.
3.3
PHANTOM IMAGE QUALITY TEST
3.3.1
Objective
To ensure that the quality of images acquired at the acquisition workstation remains adequate after the
images are transmitted to and printed on the hardcopy output device.
3.3.2
Establishing Operating Levels
The Image Receptor Manufacturer (IRM) should provide the operating levels, that is, the image quality
criteria used for scoring the printed image of the phantom.
3.3.3
Performing the QC/Constancy Test
3.3.3.1
Frequency
Annually.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
3.3.3.2
3.3.3.3
Page 24
Equipment Required
Calibrated FFDM system, including at least:
an acquisition modality (needed only if the acquisition workstation that will send clinical
images to the hardcopy device does not contain a phantom image that has recently been
acquired from the modality and that has met the image quality requirements of the image
receptor manufacturer),
an acquisition workstation
FDA-approved mammographic phantom
Procedure
Acquire an image of the accreditation phantom on a full-field digital mammography (FFDM) image
acquisition system from which images will be sent to the hardcopy device, and send the image to the
hardcopy device for printing. Alternatively, if such an image already exists on the acquisition workstation,
send that image to the hardcopy device for printing. Score the output image according to the image
quality criteria specified by the IRM. If multiple FFDM systems will be sending images to the same
hardcopy device, repeat the test for each such system.
3.3.3.4
Action Limits
The printed image must meet the image quality criteria/action limits set by the image receptor
manufacturer of each FFDM system that will send images to the hardcopy device.
3.3.3.5
Use of Test Results
If the device fails the test for a particular FFDM system from which it will receive images for printing and
final interpretation, further printing of mammograms with the device from that FFDM system must stop
until the source of the problem is identified and corrected. If the Imager passes all other applicable QC
tests described in this manual, it is likely that this image quality failure is the result of either (1) a problem
in the acquisition system or (2) a configuration issue, such as the choice of the grayscale lookup table
(i.e., Transfer Function Table (TFT)) which is being used in the imager. The first possibility may be
investigated by carefully reviewing the same acquired image on a softcopy display (preferably at a fixed
grayscale window setting) to verify that the image itself is satisfactory. The second possibility may be
investigated by switching to alternative lookup tables (TFTs), or an alternate printing mode (Presentation
LUT mode), as may be recommended by Carestream Health, Inc., especially if acquisition system
parameters have changed.
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 25
Section 4
GUIDANCE
MQSA final regulations consider performance of the image receptor manufacturers QC Test procedures
to be a requirement for compliance with the regulations. This Guidance section is not considered an
element of those regulations. It is intended to provide guidance to mammography facilities and their
personnel. It represents the equipment manufacturers current thinking on appropriate procedures for
conducting the QC tests. Procedures described in guidance represent supplementary information on
acceptable ways of doing a test, but, unlike regulations, do not bind the facility to using only those ways.
An alternative procedure may be used if such a procedure satisfies the requirements of the applicable
statute, regulations, or both. Mandatory language, such as shall, must, and require, is used when
referring to statutory or regulatory requirements. Non-mandatory language, such as should, may,
can, and recommend is used when referring to guidance. It is the responsibility of the facility to read,
understand, and follow the final regulations.
Under its own authority, a state may impose more stringent requirements than those specified under
MQSA and its implementing regulations. A facility may want to check with the state or local authorities
regarding their requirements. Tests may need to be performed more frequently than specified in this
manual if required by local regulations or the facilitys standard operating procedures.
4.1
QC TEST IMAGES
The equipment section of each QC test specifies one or more test images appropriate for that test. Table
4-1 lists some publicly available test images that can be used for this purpose.
Table 4-1
QC TEST IMAGES
Test Image
TG-18-QC
TG-18-PQC
TG-18-QC
TG-18-PQC
Source/Information/Description
AAPM Online Report #3: Assessment of Display Performance for Medical
Imaging Systems. Imaging Informatics Subcommittee Task Group #18,
and Supplemental Files
General test pattern for QC of output devices (-PQC is printer-specific)
http://www.aapm.org/pubs/reports
http://www.aapm.org/pubs/reports/public/OR_03_Supplemental/
AAPM Online Report #3: Assessment of Display Performance for Medical
Imaging Systems. Imaging Informatics Subcommittee Task Group #18,
and Supplemental Files
General test pattern for QC of output devices (-PQC is printer-specific)
http://www.aapm.org/pubs/reports
http://www.aapm.org/pubs/reports/public/OR_03_Supplemental/
SMPTE Test Pattern
SMPTE Recommended Practice RP 133-1991 - Specifications for Medical
Diagnostic Imaging Test Pattern for Television Monitors and Hard-Copy
Recording Cameras
General test pattern for QC of output devices
http://www.smpte.org/smpte_store/standards/
The test pattern only, without the Recommended Practice document, is
also downloadable from a number of Internet sources, such as:
http://brighamrad.harvard.edu/research/topics/vispercep/tutorial.html
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
4.2
Page 26
DENSITY CONSTANCY TEST (2.1)
This is a test that determines tone reproduction consistency, in other words, whether the hardcopy device
always produces the same output density (within the action limits) for a specified input gray level.
Hardcopy devices that use wet chemistry to produce an output image have more potential sources of drift
than dry printing systems, and should, therefore, be tested more frequently (daily). Dry printing systems
such as the 6850 Laser Imager also can calibrate themselves by monitoring internal parameters,
recognizing media characteristics, and adjusting their internal look-up tables to produce more consistent
results. Dry systems may have standard factory settings to which the printer should adhere, which may
obviate the need to establish operating levels (2.1.2) independently. If it is not possible to achieve these
factory settings despite repeated attempts, it is recommended that you contact your field service
engineer.
4.3
FIXER RETENTION TEST (2.2) (N/A)
This test, which is included in the NEMA template, is not applicable to dry media.
4.4
LOW-CONTRAST VISIBILITY TEST (2.3)
The importance of low-contrast targets in mammography demands a test for the reproducibility of density
differences (note that 2.1.1 is a test of the reproducibility only of selected absolute densities). For this
test, it is important that you reproduce as closely as possible the viewing conditions defined by the
manufacturer. If, after repeated attempts to visualize the low-contrast targets specified in the procedure,
the device is not performing as expected, it is recommended that you contact your field service engineer.
4.5
SPATIAL RESOLUTION TEST (2.4)
The results of the spatial resolution test are sensitive to a number of boundary conditions. One is the size
of the output medium. If the image is magnified or reduced in size to fit the output medium, the intended
results of the test may not be achieved. In a related issue, the type of interpolation used to reconstruct
the analog output image from the digital (pixel) data can have a marked effect on the reproduction of
high-spatial-frequency details. For these reasons, these parameters must be defined explicitly.
It is also important that you reproduce the viewing conditions defined by the manufacturer as closely as
possible since the 12.8 lp/mm bar pattern is normally difficult to discern in the multi-purpose QC pattern.
If the device fails this test, it is recommended first to repeat the test, paying particular attention to the
parameters cited above. If, after repeated attempts to visualize the spatial-resolution targets specified in
the procedure, the device is not performing as expected, it is recommended that you contact your field
service engineer.
4.6
(DARKROOM) FOG TEST (2.5) (N/A)
This test, which is included in the NEMA template, is not applicable to the 6850 Laser Imager since its
cartridge design prevents unintended exposure of media during normal daylight handling.
4.7
ARTIFACT TEST (2.6)
It is important to the diagnostic process that there be no artifacts in the image that could mimic or obscure
clinically significant information. However, not all possible image artifacts fall into this category. For
example, low levels of streaks or bands, minus density points, or test pattern ghosting may not affect the
diagnostic process.
Ghosting artifacts observed in the TG18-QC Pattern could occur at black to gray transitions. The TG18QC image has two large areas of the pattern that are set to 0 pixel value (or 0%) and occur over long
excursions that suddenly increase into long excursions of 50% pixel value. One is the Cross Talk bars
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 27
and the other is the Cx background. Extended areas of maximum density which occur through or near an
adjacent 50% gray background in an image could result in a ghost streak observed within the gray field.
Extended areas of maximum density that abruptly transition to gray do not occur in mammography
images. Therefore the appearance of ghosting in the TG-18QC pattern at these transitions is not
predictive of diagnostic concerns in the printed film.
It will be helpful to use the concept of ALARA (as low as reasonably achievable) when judging artifacts.
The medical physicist should consult with the interpreting physician as to whether an artifact is tolerable
by comparing a clinical image to the film with the noted artifact.
4.8
GRAYSCALE RESPONSE FUNCTION TEST (3.1)
The Density Constancy Test (2.1) checks the tone reproduction consistency only at a few key output
densities. The Grayscale Response Function test verifies that the tone reproduction curve conforms
(within the action limits) to an established reference curve. Such reference curves can be manufacturerdependent, and can be tuned to a particular application based on clinical testing, or other criteria. Other
curves, such as the DICOM Grayscale Standard Display Function (DICOM Part 3.14) have been
proposed as universal reference curves that mimic human visual response to luminance.
A hardcopy device such as the 6850 Laser Imager is designed to recalibrate itself when necessary (for
example, when the film lot number changes) to return to the desired curve.
The actual curve used in clinical image printing can be different from the curve used in this test. It can be
chosen from a large number of available curves. For the 6850 Laser Imager, any of a large number of
Transfer Function Tables (TFTs) or User Look-Up Tables (ULUTs) can be selected. Some of these are
based on the DICOM Grayscale Standard Display Function (GSDF), tailored for various combinations of
Dmax, lightbox luminance, and reflected ambient light. It is also possible to print in the DICOM
Presentation Lookup Table (PLUT) mode, which allows GSDF-based response based on density and
viewing parameters specified by the printing modality.
4.9
GEOMETRY TEST (3.2)
Geometric distortion of image features can lead to misleading information about clinical structures and
pathology. This test requires accurate measurements of distances between specific landmarks on the
test image. Make sure that the measurement tool (preferably, a metal ruler) has sufficient accuracy and
precision for the task.
4.10
PHANTOM IMAGE QUALITY TEST (3.3)
This is the only system test included in the QC manual: it tests the transfer of information from the
acquisition modality through to the output device. If the hardcopy device fails the Phantom Image Quality
Test, ensure that all other elements of the imaging chain are working as intended, and repeat the test. If
the system still fails the test, it is recommended that you contact the appropriate field service engineer.
4.11
MAMMOGRAPHY EQUIPMENT EVALUATION (MEE)
Sections 2 and 3 describe periodic QC tests. There are other occasions when QC/acceptance tests must
be done ad hoc after interventions that might affect imaging performance. These include changes or
repairs to the hardcopy devices exposure and processing hardware components and software updates,
or the installation of a new host device. In order to verify that all functions that may have been affected by
the change/repair have been successfully restored/initialized, the hardcopy output device must pass a
mammography equipment evaluation (MEE). MEEs shall be performed by a medical physicist or by an
individual under the direct supervision of a medical physicist and all problems shall be corrected before
the new or changed equipment is put into service for examinations... (21 CFR 900.12(e)(10)).
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
DRYVIEW 6850 Laser Imager, Quality Control Manual for Mammography
Page 28
If the MEE is for the purpose of evaluating/accepting a newly installed hardcopy output device, first verify
that the installer has completed all tasks defined by the manufacturer as necessary for proper installation.
These include:
Out-of-box equipment inspection
Equipment setup
Equipment configuration
Equipment verification testing
Once the hardcopy device is functional and communicating with the rest of the FFDM system, the MEE
consists of the following tests:
MEE Test Name
Section
Density Constancy
2.1
Low-contrast Visibility
2.3
Spatial Resolution
2.4
Artifact
2.6
Grayscale Response Function
3.1
Geometry
3.2
Phantom Image Quality
3.3
DRYVIEW is a trademark of Carestream Health, Inc.
150 Verona Street
Rochester, NY 14608 USA
Printed in U.S.A. 05/10
Carestream Health, Inc., 2010
Carestream Health, Inc. May 11, 2010
8H5323 Rev. A
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- Manual QDR Software PDFDocumento694 pagineManual QDR Software PDFJose Quisca100% (1)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Vacora IFU MultilingueDocumento84 pagineVacora IFU MultilingueJose QuiscaNessuna valutazione finora
- Tune Up (System)Documento104 pagineTune Up (System)Jose Quisca100% (2)
- DryPix Plus 4000 Reference GuideDocumento174 pagineDryPix Plus 4000 Reference GuideJose Quisca80% (5)
- Lesson Plan in Science 7Documento1 paginaLesson Plan in Science 7ma. melanie s. capawing100% (9)
- Measuring Cells LabDocumento3 pagineMeasuring Cells LabClaudia100% (2)
- RaySafe Xi Manual en MDocumento49 pagineRaySafe Xi Manual en MJose QuiscaNessuna valutazione finora
- Carestream drx-1Documento7 pagineCarestream drx-1Jose QuiscaNessuna valutazione finora
- Raysafe Xi View: User ManualDocumento15 pagineRaysafe Xi View: User ManualJose QuiscaNessuna valutazione finora
- Flat Panel 1417 - RAYENCEDocumento2 pagineFlat Panel 1417 - RAYENCEJose QuiscaNessuna valutazione finora
- E-CUBE 9 Catalog - Internal MedicineDocumento4 pagineE-CUBE 9 Catalog - Internal MedicineJose QuiscaNessuna valutazione finora
- E-Cube 9 Diamond CatalogDocumento14 pagineE-Cube 9 Diamond CatalogJose QuiscaNessuna valutazione finora
- E-CUBE 9 Catalog - MusculoskeletalDocumento4 pagineE-CUBE 9 Catalog - MusculoskeletalJose QuiscaNessuna valutazione finora
- E-CUBE 9 Catalog - Womans HealthDocumento6 pagineE-CUBE 9 Catalog - Womans HealthJose QuiscaNessuna valutazione finora
- Lorad Sel QCMan Rev 7Documento146 pagineLorad Sel QCMan Rev 7Jose Quisca100% (2)
- Usuario Dryview 6850Documento46 pagineUsuario Dryview 6850Jose QuiscaNessuna valutazione finora
- Flyer X-RayDocumento4 pagineFlyer X-RayJose QuiscaNessuna valutazione finora
- DXA Radiation Dose ComparisonDocumento2 pagineDXA Radiation Dose ComparisonJose QuiscaNessuna valutazione finora
- AA3426 - SPT1 User Guide - 2013-04-08 - enDocumento26 pagineAA3426 - SPT1 User Guide - 2013-04-08 - enJose Quisca100% (1)
- Engineering Change Notice (Field Change Notice) : ECN No. 2010-E-0234Documento2 pagineEngineering Change Notice (Field Change Notice) : ECN No. 2010-E-0234Jose QuiscaNessuna valutazione finora
- CrSoftwareV4-6 Mds2 8H3154Documento3 pagineCrSoftwareV4-6 Mds2 8H3154Jose QuiscaNessuna valutazione finora
- EcoView 9 Plus-12p SmallDocumento6 pagineEcoView 9 Plus-12p SmallJose Quisca0% (1)
- Agfa CR 30 XDocumento31 pagineAgfa CR 30 XLuis Fernando Garcia SNessuna valutazione finora
- SamsungXmaru1717 Prospektblatt EDocumento2 pagineSamsungXmaru1717 Prospektblatt EJose Quisca100% (1)
- E-Cube Line Up CatalogDocumento16 pagineE-Cube Line Up CatalogJose QuiscaNessuna valutazione finora
- A Step-By-Step Guide To Burn A CD in Efilm: Step 1: Copy An Entire StudyDocumento2 pagineA Step-By-Step Guide To Burn A CD in Efilm: Step 1: Copy An Entire StudyJose QuiscaNessuna valutazione finora
- AccuStitch 114 Users Guide For EFilm Workstation VeterinaryDocumento52 pagineAccuStitch 114 Users Guide For EFilm Workstation VeterinaryJose QuiscaNessuna valutazione finora
- Ref Guide 959 PDFDocumento2 pagineRef Guide 959 PDFJose QuiscaNessuna valutazione finora
- Sound Eklin Efilm Tools Quick Reference Guide 092311Documento1 paginaSound Eklin Efilm Tools Quick Reference Guide 092311Jose QuiscaNessuna valutazione finora
- 897N0602G AspireCRm QC Manual PDFDocumento106 pagine897N0602G AspireCRm QC Manual PDFJose QuiscaNessuna valutazione finora
- Cawomat 2000IR - SchematicsDocumento1 paginaCawomat 2000IR - SchematicsJose QuiscaNessuna valutazione finora
- Physics Formula SheetDocumento13 paginePhysics Formula Sheetthunder boltNessuna valutazione finora
- Science Class X Term I Reference Study Material 166 169Documento4 pagineScience Class X Term I Reference Study Material 166 169Alina SaraswatNessuna valutazione finora
- Jupiter Portable Magnifier User ManualDocumento26 pagineJupiter Portable Magnifier User ManualalguienjoshepNessuna valutazione finora
- Scheme of Work - FM 4 PhysicsDocumento27 pagineScheme of Work - FM 4 PhysicsAnonymous aiinSGRwsNessuna valutazione finora
- 'Optical Instruments and Their Function''Documento8 pagine'Optical Instruments and Their Function''Acé Vínce MontéverdeNessuna valutazione finora
- Basics of Light Microscopy ImagingDocumento55 pagineBasics of Light Microscopy Imagingqazim786Nessuna valutazione finora
- Opmi Proergo BrochureDocumento8 pagineOpmi Proergo BrochureMiriam SanchezNessuna valutazione finora
- Wolkite University College of Natural and Computational Sciences Department of BiologyDocumento155 pagineWolkite University College of Natural and Computational Sciences Department of BiologyJimach Bol WieNessuna valutazione finora
- Submitted By: Carlo A. Toledo Submitted To: Ms. Bea Magno: 12-STEM CDocumento6 pagineSubmitted By: Carlo A. Toledo Submitted To: Ms. Bea Magno: 12-STEM CCarlo ToledoNessuna valutazione finora
- Carl Zeiss Jena Apo TessarDocumento28 pagineCarl Zeiss Jena Apo TessarElla Antonella0% (1)
- Distance Learning Programme: Jee (Main) : Leader Test Series / Joint Package CourseDocumento52 pagineDistance Learning Programme: Jee (Main) : Leader Test Series / Joint Package CourseSaptarshiNessuna valutazione finora
- Industrial Borescope: Operating ManualDocumento16 pagineIndustrial Borescope: Operating ManualFAISHAH ISAHAKNessuna valutazione finora
- Skema Trial Kelantan 2010 Physics Edit 1Documento14 pagineSkema Trial Kelantan 2010 Physics Edit 1lilysuhanyNessuna valutazione finora
- What I Need To Know?: PipetteDocumento3 pagineWhat I Need To Know?: Pipetteamora eliNessuna valutazione finora
- .Documento56 pagine.Ioana Maria AvramNessuna valutazione finora
- ElectrostaticsDocumento11 pagineElectrostaticsashish aryaNessuna valutazione finora
- Regal Christmas 2009Documento118 pagineRegal Christmas 2009The Catalogue Stop100% (1)
- Review Notes in Physics 2-ECEDocumento12 pagineReview Notes in Physics 2-ECEAnonymous cuVSFiNessuna valutazione finora
- 7 MicrometryDocumento13 pagine7 MicrometryChai Kah ChunNessuna valutazione finora
- Vividia® Ablescope® Viewer For Windows User GuideDocumento27 pagineVividia® Ablescope® Viewer For Windows User GuidearshadNessuna valutazione finora
- SUMMARY of Physics 9-10Documento44 pagineSUMMARY of Physics 9-10freeuser3Nessuna valutazione finora
- Fiitjee: Solutions To JEE (Main) - 2021Documento41 pagineFiitjee: Solutions To JEE (Main) - 2021ik62299Nessuna valutazione finora
- Avian EndosDocumento15 pagineAvian EndosJosé Moreira Lima NetoNessuna valutazione finora
- B1, Topic 1.1 Student Practical: Observation of Cells Under A MicroscopeDocumento5 pagineB1, Topic 1.1 Student Practical: Observation of Cells Under A MicroscopeFatimah MNessuna valutazione finora
- Letter e Lab - HDocumento2 pagineLetter e Lab - HDean JezerNessuna valutazione finora
- How Digital Cameras WorkDocumento17 pagineHow Digital Cameras WorkAnusha DandayNessuna valutazione finora
- Biology Experiments For ChildrenDocumento95 pagineBiology Experiments For Childrentapas kunduNessuna valutazione finora
- NDT-Basic-FormulaeDocumento3 pagineNDT-Basic-FormulaeJayeshNessuna valutazione finora