Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Non-Ideal Solutions: Strong Deviations From Ideality Are Shown by Dissimilar Substances
Caricato da
mallikapathakDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Non-Ideal Solutions: Strong Deviations From Ideality Are Shown by Dissimilar Substances
Caricato da
mallikapathakCopyright:
Formati disponibili
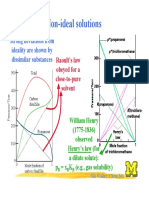
Non-ideal solutions
Strong deviations from
ideality are shown by
dissimilar substances Raoults law
obeyed for a
close-to-pure
solvent
William Henry
(1775-1836)
observed
Henrys law (for
a dilute solute):
pB = xBKB (e.g., gas solubility)
Nils Walter: Chem 260
Ideal and real solutions: Activities
From both Raoults (solvent) and
Henrys laws (solute) follows:
O
solv (l ) = solv
(l ) + RT ln xsolv
O
(l ) + RT ln C[ solv ]
= solv
O
J
J + RT ln[J ]
standard chemical
potential @ 1 M
The chemical potential is a
measure of the ability of J
to bring about physical or
chemical change
BUT:
to
preserve
equation
for real
solutions:
J = OJ + RT ln a J
Effective concentration
= activity aJ = J[J]
Nils Walter: Chem 260
Consequences of chemical potential changes
in mixtures: Colligative properties
Freezing point depression:
Tf = KfbB
Boiling point elevation:
TB = KBbB
cryoscopic constant
Solute is insoluble
in solid solvent:
ebullioscopic constant
molality
Solute is not
volatile:
Chemical
Potential
lowered by
solute
Chemical
Potential
lowered by
solute
Nils Walter: Chem 260
Phase diagrams of binary mixtures
Phase rule: F = C - P + 2
p = constant:
p,T
for binary mixtures = 2
Temperature-composition diagram
for binary mixture of volatile liquids
in
equilibrium
Nils Walter: Chem 260
Finally, as promised: Whisky distillery
Fractional distillation:
Non-ideal mixtures
High-boiling azeotrope,
e.g., nitric acid/water
Low-boiling azeotrope,
e.g., ethanol/water
Nils Walter: Chem 260
Liquid-liquid phase diagrams of
partially miscible liquids
E.g., hexane/nitrobenzene:
Upper critical
solution
temperature
(thermal motion)
E.g., triethylamine
/water:
Lever rule
Lower Tcrit
(complex formed)
Amount of phase of composition a" l '
Nils Walter: Chem 260 =
Amount of phase of composition a' l"
Potrebbero piacerti anche
- Non-Ideal Solutions: Strong Deviations From Ideality Are Shown by Dissimilar SubstancesDocumento4 pagineNon-Ideal Solutions: Strong Deviations From Ideality Are Shown by Dissimilar SubstancesHamed NazariNessuna valutazione finora
- Non-Ideal Solutions: Strong Deviations From Ideality Are Shown by Dissimilar SubstancesDocumento6 pagineNon-Ideal Solutions: Strong Deviations From Ideality Are Shown by Dissimilar SubstancesrjslNessuna valutazione finora
- Equillibrium RevisionDocumento6 pagineEquillibrium RevisionMahesh BabuNessuna valutazione finora
- Chem 17 Finals ReviewerDocumento9 pagineChem 17 Finals ReviewerJamie Joice Noche100% (1)
- Chem 30 Course Summary 4Documento10 pagineChem 30 Course Summary 4dutritinh0806Nessuna valutazione finora
- Ch. 6: Chemical Equilibrium: Updated Oct. 5, 2011: Minor Fix On Slide 11, New Slides 31-42Documento42 pagineCh. 6: Chemical Equilibrium: Updated Oct. 5, 2011: Minor Fix On Slide 11, New Slides 31-42Ankit RawatNessuna valutazione finora
- Chemical Equilibrium in Analytical ChemistryDocumento42 pagineChemical Equilibrium in Analytical Chemistryalex tomsonNessuna valutazione finora
- Molecular Description of Dissolution: SolubilityDocumento10 pagineMolecular Description of Dissolution: SolubilityRaaz Murdoc GurungNessuna valutazione finora
- BiochemPrep1 PDFDocumento49 pagineBiochemPrep1 PDFShixia XuNessuna valutazione finora
- Chapter 06Documento44 pagineChapter 06makroniNessuna valutazione finora
- Equilibrium Equilibrium in Physical ProcessesDocumento6 pagineEquilibrium Equilibrium in Physical ProcessesSteveMathewKuruvillaNessuna valutazione finora
- MolarityDocumento7 pagineMolarityMacxieNessuna valutazione finora
- Chemical Equilibrium .PresentationDocumento17 pagineChemical Equilibrium .PresentationtalhawasimNessuna valutazione finora
- Chemical Equilibrium GuideDocumento44 pagineChemical Equilibrium GuideSteph Kier PonterasNessuna valutazione finora
- Solubility 2010 PDFDocumento10 pagineSolubility 2010 PDFbadri parthasaradhiNessuna valutazione finora
- Articulo Equilibrioquimico 19661Documento4 pagineArticulo Equilibrioquimico 19661dexgigiNessuna valutazione finora
- Lecture28 PDFDocumento5 pagineLecture28 PDFAdwaithGopanNessuna valutazione finora
- Capsule For Low AchieversDocumento17 pagineCapsule For Low AchieversPratham Zala100% (1)
- Solutions and Chemical Kinetics GuideDocumento11 pagineSolutions and Chemical Kinetics GuideKADAMBARINessuna valutazione finora
- 16 EquilibDocumento43 pagine16 Equilib01921386384Nessuna valutazione finora
- Physical Chemistry EquationsDocumento2 paginePhysical Chemistry Equationsvixi_hNessuna valutazione finora
- Equilibrium ConstantDocumento26 pagineEquilibrium ConstantFarooq AhmadNessuna valutazione finora
- Efek Panas PD Proses Pencampuran - Smith Vaness 6th-441-454Documento14 pagineEfek Panas PD Proses Pencampuran - Smith Vaness 6th-441-454florentinaNessuna valutazione finora
- Formula Sheet ThermodynamicsDocumento14 pagineFormula Sheet ThermodynamicstcbrihzllmkmkvxfovNessuna valutazione finora
- Chapter 31Documento80 pagineChapter 31Laila UbandoNessuna valutazione finora
- Experiment 13 Post LabDocumento40 pagineExperiment 13 Post LabEmill Jayson CaypunoNessuna valutazione finora
- Enthalpy ChangesSMPLFDDocumento55 pagineEnthalpy ChangesSMPLFDtopherchandraNessuna valutazione finora
- Module 4Documento19 pagineModule 4Sanjeeb SutradharNessuna valutazione finora
- Entropy and Gibbs Free Energy Explained in 40 CharactersDocumento6 pagineEntropy and Gibbs Free Energy Explained in 40 CharactersZyriel SaavedraNessuna valutazione finora
- Elimination ReactionDocumento5 pagineElimination ReactionpreethaNessuna valutazione finora
- CH 14Documento75 pagineCH 14Pavi ChariNessuna valutazione finora
- Le Chatelier's Principle Flashcards - QuizletDocumento4 pagineLe Chatelier's Principle Flashcards - QuizletAsiff MohammedNessuna valutazione finora
- EquilibriumDocumento5 pagineEquilibriumnanaNessuna valutazione finora
- Solutions NCERTDocumento16 pagineSolutions NCERTPrecisive OneNessuna valutazione finora
- Equilibrium Class 11th Notes Mind Map and MCQ Cbse Chemistry 0 2024 09 01 084331Documento41 pagineEquilibrium Class 11th Notes Mind Map and MCQ Cbse Chemistry 0 2024 09 01 084331rashmiNessuna valutazione finora
- Chemical Kinetics: Medical ChemistryDocumento46 pagineChemical Kinetics: Medical ChemistryВиталий НечипорукNessuna valutazione finora
- Le Chatelier's Principle:: Chemistry Study GuideDocumento4 pagineLe Chatelier's Principle:: Chemistry Study GuideBrielle AndersonNessuna valutazione finora
- Inorganic Chemistry IIDocumento23 pagineInorganic Chemistry IIAlvin Garcia PalancaNessuna valutazione finora
- Wa0025.Documento7 pagineWa0025.Uday BhaskarNessuna valutazione finora
- Chapter 15Documento32 pagineChapter 15Dana CapbunNessuna valutazione finora
- Environmental Chemistry and Microbiology: NptelDocumento57 pagineEnvironmental Chemistry and Microbiology: NptelAbhijit NathNessuna valutazione finora
- Chemical EqulibriumDocumento23 pagineChemical Equlibriumiqbal-cheNessuna valutazione finora
- CHM 101 Lecture Note On Thermo Chemical KineticsDocumento24 pagineCHM 101 Lecture Note On Thermo Chemical Kineticsekanadefestus007Nessuna valutazione finora
- 2-Excellent Chemistry Assignment SolutionsDocumento5 pagine2-Excellent Chemistry Assignment SolutionsSachin B SNessuna valutazione finora
- Document PDFDocumento9 pagineDocument PDFFF ROCKEYNessuna valutazione finora
- IntroductionDocumento15 pagineIntroductionOP IMPERIALNessuna valutazione finora
- SolubilityDocumento37 pagineSolubilityLalitha Sravani100% (1)
- CH 15 - Chemical Equilibrium Compatibility ModeDocumento16 pagineCH 15 - Chemical Equilibrium Compatibility ModeIcha Meisyarani IINessuna valutazione finora
- Chemical Equilibrium: Heterogeneous and Homogeneous Equilibrium Law of Mass ActionDocumento20 pagineChemical Equilibrium: Heterogeneous and Homogeneous Equilibrium Law of Mass ActionVíctor RuedaNessuna valutazione finora
- Chemistry 2Documento17 pagineChemistry 2Harshit ChoudharyNessuna valutazione finora
- Thermodynamics (Part 2)Documento31 pagineThermodynamics (Part 2)Gabriel DiuyanNessuna valutazione finora
- Quick Revision CapsuleDocumento18 pagineQuick Revision CapsuleRacsGamer100% (1)
- Chemical Equlibrium (Autosaved)Documento21 pagineChemical Equlibrium (Autosaved)iqbal-cheNessuna valutazione finora
- Structure and Reactivity: Acids and Bases, Polar and Nonpolar MoleculesDocumento53 pagineStructure and Reactivity: Acids and Bases, Polar and Nonpolar MoleculesAdzimahNessuna valutazione finora
- Thermodynamics and Equilibrium Class NotesDocumento40 pagineThermodynamics and Equilibrium Class NotesDotan NutodNessuna valutazione finora
- Prinsip Le Chatelier Dan KatalisisDocumento19 paginePrinsip Le Chatelier Dan KatalisisLestari zaiNessuna valutazione finora
- CHPR4406 Reactions Lecture 1Documento16 pagineCHPR4406 Reactions Lecture 1xx_aleksa_hrvatska_xxNessuna valutazione finora
- Free-Radical Chain ReactionDocumento36 pagineFree-Radical Chain ReactionRahma FatmawatiNessuna valutazione finora
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsDa EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNessuna valutazione finora
- Phase Equilibrium II: - Two Component SystemDocumento41 paginePhase Equilibrium II: - Two Component SystemAnindya Ajeng PrameswariNessuna valutazione finora
- Moodle Write UpDocumento3 pagineMoodle Write Upmallikapathak100% (1)
- Principa L: Administrative Officer Bursar Vice Principal Administra Tion Accoun Ts Hostel Library Departme NtsDocumento1 paginaPrincipa L: Administrative Officer Bursar Vice Principal Administra Tion Accoun Ts Hostel Library Departme NtsmallikapathakNessuna valutazione finora
- 15 Chem KinetDocumento51 pagine15 Chem Kinetaby251188Nessuna valutazione finora
- Dist PLDocumento11 pagineDist PLTalal AshrafNessuna valutazione finora
- Kinetics Books Ta VerDocumento63 pagineKinetics Books Ta VermallikapathakNessuna valutazione finora
- MH Alumni-3Documento3 pagineMH Alumni-3mallikapathakNessuna valutazione finora
- Chapter 14auLectureSlides 000Documento123 pagineChapter 14auLectureSlides 000Eule100Nessuna valutazione finora
- Kinetics Books Ta VerDocumento63 pagineKinetics Books Ta VermallikapathakNessuna valutazione finora
- Thermodynamics of Separation Operations ExplainedDocumento21 pagineThermodynamics of Separation Operations ExplainedgongweejieNessuna valutazione finora
- Electrochemistry TestDocumento1 paginaElectrochemistry TestmallikapathakNessuna valutazione finora
- Water PollutionDocumento10 pagineWater PollutionsonuraaNessuna valutazione finora
- Msds PresentationDocumento20 pagineMsds PresentationmallikapathakNessuna valutazione finora
- Lab Safety Rules Poster PDFDocumento1 paginaLab Safety Rules Poster PDFmallikapathak50% (2)
- G-6 PH of Soaps - Himashree and GroupDocumento14 pagineG-6 PH of Soaps - Himashree and Groupmallikapathak100% (1)
- Plant Materials: Indica) (Terminalia Chebula) Granatum Catechu) Inermis)Documento1 paginaPlant Materials: Indica) (Terminalia Chebula) Granatum Catechu) Inermis)mallikapathakNessuna valutazione finora
- Electrochemistry TestDocumento1 paginaElectrochemistry TestmallikapathakNessuna valutazione finora
- 36x48 Template V3Documento1 pagina36x48 Template V3mallikapathakNessuna valutazione finora
- BrochureDocumento2 pagineBrochuremallikapathakNessuna valutazione finora
- 16april FinalDocumento13 pagine16april FinalmallikapathakNessuna valutazione finora
- Research Poster 1Documento1 paginaResearch Poster 1mallikapathakNessuna valutazione finora
- BSC I Physics Tests For Pracs'09Documento3 pagineBSC I Physics Tests For Pracs'09mallikapathakNessuna valutazione finora
- Detection of Extra ElementsDocumento77 pagineDetection of Extra Elementsmallikapathak80% (5)
- C142 Exp10Documento3 pagineC142 Exp10mallikapathakNessuna valutazione finora
- Lecture 2-Why Treat Water ?Documento24 pagineLecture 2-Why Treat Water ?Harold TaylorNessuna valutazione finora
- Redox Tit Rations Only - FinalDocumento16 pagineRedox Tit Rations Only - FinalpolamrajuNessuna valutazione finora
- Manual 3322006Documento46 pagineManual 3322006mallikapathakNessuna valutazione finora
- C141 Exp2Documento4 pagineC141 Exp2mallikapathakNessuna valutazione finora