Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Essential Standards Chart - CH 11
Caricato da
api-313496561Descrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Essential Standards Chart - CH 11
Caricato da
api-313496561Copyright:
Formati disponibili
EssentialStandardsChart:Whatisitweexpectstudentstolearn?
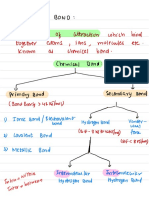
Standard:Atomsformbondsbytheinteractionoftheirvalenceelectrons
Grade:
11
Subject:
TargetGoalDescription
Whatisthetargetgoaltobe
learned?Describeinstudent
friendlyvocabulary.
Chemistry
Semester
Team
Members:
ExampleRigor
PrerequisiteSkills
Common
Assessment
WhenTaught?
Extension
Standards
Whatdoesproficientstudentworklooklike?
Provideanexampleand/ordescription.
Whatpriorknowledge,
skills,and/orvocabulary
is/areneededfora
studenttomasterthis
standard?
Whatassessment(s)will
beusedtomeasure
studentmastery?
Whenwillthis
standard
betaught?
Whatwillwedo
whenstudents
havelearnedthe
essential
standard(s)?
Ionic bonding
Test
February
Lewis dot structures
for atoms
Test & Lab over
structures
February
Nomenclature for
ionic bonding
Test
February
Explain how atoms
combine to form
Students understand electrons are
compounds through both shared to make bonds and can identify
ionic and covalent
sigma and pi bonds
bonding
Draw Lewis dot
structures for simple
molecules
Name and write the
chemical formulas for
simple ionic and
molecular compounds,
including those that
contain common
polyatomic ions
N2O3 dinitrogen trioxide
Buffum/Mattos/Weber, 2011
Buffum/Mattos/Weber, 2011
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Chemical Bonding and Lewis StructureDocumento26 pagineChemical Bonding and Lewis StructureCassandra Nicole SalasinaNessuna valutazione finora
- Unit 6: Ionic & Covalent Compounds, Lewis Structures: Chapter 5 & 6Documento38 pagineUnit 6: Ionic & Covalent Compounds, Lewis Structures: Chapter 5 & 6chanNessuna valutazione finora
- Chemical Bonding Igcse Only Complete 2014 ML PDFDocumento88 pagineChemical Bonding Igcse Only Complete 2014 ML PDFAli AshrafNessuna valutazione finora
- Abuse TrainingDocumento1 paginaAbuse Trainingapi-313496561Nessuna valutazione finora
- Adi Lab 7 - Periodic TrendsDocumento3 pagineAdi Lab 7 - Periodic Trendsapi-313496561Nessuna valutazione finora
- Sciencebap2015 2016Documento5 pagineSciencebap2015 2016api-313496561Nessuna valutazione finora
- Essential Standards Chart - CH 11Documento2 pagineEssential Standards Chart - CH 11api-313496561Nessuna valutazione finora
- Safety ContractDocumento2 pagineSafety Contractapi-313496561Nessuna valutazione finora
- Mannich Reaction SamplesDocumento5 pagineMannich Reaction Samplesapi-313496561Nessuna valutazione finora
- Dbcs - Biorenewables Vs NonrenewablesDocumento8 pagineDbcs - Biorenewables Vs Nonrenewablesapi-313496561Nessuna valutazione finora
- Mannichreactions IsuDocumento1 paginaMannichreactions Isuapi-313496561Nessuna valutazione finora
- Lab Safety ProceduresDocumento7 pagineLab Safety Proceduresapi-313496561Nessuna valutazione finora
- Ret Poster 2015Documento1 paginaRet Poster 2015api-313496561Nessuna valutazione finora
- Kmuyskensipdp 2013Documento5 pagineKmuyskensipdp 2013api-313496561Nessuna valutazione finora
- Intro To Chemistry Chapter 11 Part 1 Test: H S CH CODocumento1 paginaIntro To Chemistry Chapter 11 Part 1 Test: H S CH COapi-313496561Nessuna valutazione finora
- ChemistrysyllabusDocumento2 pagineChemistrysyllabusapi-313496561Nessuna valutazione finora
- Essential Standards Chart - CH 11Documento2 pagineEssential Standards Chart - CH 11api-313496561Nessuna valutazione finora
- Essential Standards Chart - CH 11Documento2 pagineEssential Standards Chart - CH 11api-313496561Nessuna valutazione finora
- Test ch11 5Documento2 pagineTest ch11 5api-313496561Nessuna valutazione finora
- Aiwscoringsciencetask 10Documento1 paginaAiwscoringsciencetask 10api-313496561Nessuna valutazione finora
- CH 4 Chemistry GridDocumento1 paginaCH 4 Chemistry Gridapi-313496561Nessuna valutazione finora
- ChemistrysyllabusDocumento2 pagineChemistrysyllabusapi-313496561Nessuna valutazione finora
- The Chemistry of Coordination CompoundsDocumento33 pagineThe Chemistry of Coordination CompoundsRiandy PutraNessuna valutazione finora
- Mineral ChemistryDocumento41 pagineMineral ChemistryLeandro OliveiraNessuna valutazione finora
- General Chemistry 1: Groups in The Periodic TableDocumento4 pagineGeneral Chemistry 1: Groups in The Periodic TableShane G.Nessuna valutazione finora
- Inorganic Paper III 2010Documento47 pagineInorganic Paper III 2010ls1955100% (3)
- W.S 2.2 Answer KeyDocumento3 pagineW.S 2.2 Answer Keythreefold18 -BRAWL STARSNessuna valutazione finora
- G9.MODULE 2 Lesson 6.2 Formation of IonsDocumento10 pagineG9.MODULE 2 Lesson 6.2 Formation of IonsAndrina Binogwal TocgongnaNessuna valutazione finora
- Intermolecular ForcesDocumento14 pagineIntermolecular ForcesFrancis SullanoNessuna valutazione finora
- 13.periodic Table and Periodicity PDFDocumento20 pagine13.periodic Table and Periodicity PDFP. E. I. AcademicsNessuna valutazione finora
- Reaction IntermediatesDocumento5 pagineReaction Intermediatescybercp100% (1)
- Review of IB Chem Topics 4 and 14Documento10 pagineReview of IB Chem Topics 4 and 14coolpianocatNessuna valutazione finora
- Chapter 9 Ism 11e FinalDocumento29 pagineChapter 9 Ism 11e FinalNathan VitorNessuna valutazione finora
- Lesson 2 Electron and Lewis StructureDocumento19 pagineLesson 2 Electron and Lewis StructurezzzzzNessuna valutazione finora
- Chemistry 1251 Practice Exam SolutionsDocumento10 pagineChemistry 1251 Practice Exam SolutionssmithNessuna valutazione finora
- VSEPRDocumento44 pagineVSEPRAhmad NaumanNessuna valutazione finora
- Student Exploration: Covalent BondsDocumento6 pagineStudent Exploration: Covalent BondsJenayaNessuna valutazione finora
- CHEMICAL BONDING. Revision WorksheetDocumento3 pagineCHEMICAL BONDING. Revision WorksheetLalithNessuna valutazione finora
- 연습문제 SolutionDocumento156 pagine연습문제 Solution박민지Nessuna valutazione finora
- MOT Inorganic: Dr. Sajjad Hussain Sumrra (CHEM-305) Chemistry-IIDocumento52 pagineMOT Inorganic: Dr. Sajjad Hussain Sumrra (CHEM-305) Chemistry-IITanya Dilshad100% (2)
- Covalent Bonding NotesDocumento39 pagineCovalent Bonding NotesAmaris HopkinsNessuna valutazione finora
- 11 Chemistry Notes Chapter 3Documento13 pagine11 Chemistry Notes Chapter 3Gyani ChachaNessuna valutazione finora
- Chemical Bond: The Force that Binds Atoms TogetherDocumento15 pagineChemical Bond: The Force that Binds Atoms TogetherShreyas PrabhuNessuna valutazione finora
- Answer Key For Chemical BondsDocumento2 pagineAnswer Key For Chemical Bondsapi-286297901Nessuna valutazione finora
- Answer Bank ct-1Documento9 pagineAnswer Bank ct-1MAHESHWAR M R (RA2111004010136)Nessuna valutazione finora
- Module 4Documento2 pagineModule 4Christopher Agustin Tambogon LptNessuna valutazione finora
- SCIENCE 9 UNIT PLAN Quarter 2Documento6 pagineSCIENCE 9 UNIT PLAN Quarter 2Janice Paje100% (1)
- SL MC Test s2 Models of Bonding - Structure (Second Test)Documento7 pagineSL MC Test s2 Models of Bonding - Structure (Second Test)Amira Selpa KhairunnisaNessuna valutazione finora
- Chemistry Test 5 Study GuideDocumento3 pagineChemistry Test 5 Study GuideLeanne RoseNessuna valutazione finora