Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Heterocycles - PART 2 - Pall Knorr Pyrrole
Caricato da
Ghadeer M Hassan0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
6 visualizzazioni4 paginet5
Copyright
© © All Rights Reserved
Formati disponibili
PPTX, PDF o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentot5
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
6 visualizzazioni4 pagineHeterocycles - PART 2 - Pall Knorr Pyrrole
Caricato da
Ghadeer M Hassant5
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF o leggi online su Scribd
Sei sulla pagina 1di 4
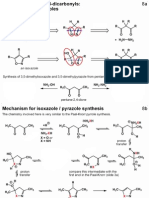
Heterocycles from 1,4-diketones: the Paal-Knorr synthesis 2b
When hexan-2,5-dione is treated with base an aldol-dehydration takes place, via the enolate, yielding
an a,B-unsaturated ketone (3-methylcyclopentenone). Treatment with a strong acid however yields a
furan (2,5-dimethylfuran) via the enol
eo ®
eee weeny 2
-H,0
-H20 oo
Related reactions under acidic conditions can yield pyrroles and thiophenes.
Hs, 4°
Rt R20
®
< RNH2, H
Rt Re —__»
Mechanism of the Paal-Knorr synthesis
3a
H
®
we ex, mu we om
°
vd Ss,
proton
\ transfer \
H3C’ 0 CH3 H3C" ‘CH
H,0
©
-H,0
H.
conditions of thermodynamic
control. The stable aromatic
furan is ultimately produced.
The reaction proceeds under
oe
Hse"Ng Hy HC CH3
©
Paal-Knorr synthesis: pyrroles and thiophenes 3b
The chemistry involved here is essentially the same as the furan example before, but an enamine or
thioenol intermediate is needed
XH ©
© ar) XH,
wo yom eo H3C- oH wok Loi,
0 Oo 0 0 = = 0°
n X=NR,X=S \
ON 7
proton
transfer
enamine (X=NR) or thioenol (X=S)
H
4® XH
: H,C: CH3 wo Lon,
Oo Xx. 0 0®
OM oH
\ M22 Ok
woX Sox, neh dom SF eA Hs
> % HO 7®
Paal-Knorr synthesi:
: examples 4a
Work out the products from the following reactions:
HPO, NH, HO
Potrebbero piacerti anche
- Adrenocorticoids2- ادوية نظري - د.احسانDocumento4 pagineAdrenocorticoids2- ادوية نظري - د.احسانGhadeer M HassanNessuna valutazione finora
- Quinoline SynthesisDocumento6 pagineQuinoline SynthesisFrancesco TutinoNessuna valutazione finora
- Acs سريرية نظري- د.حيدرDocumento56 pagineAcs سريرية نظري- د.حيدرGhadeer M HassanNessuna valutazione finora
- Compartment ModelsDocumento67 pagineCompartment ModelsGhadeer M HassanNessuna valutazione finora
- 2004 OrgSeminarTestAnswerDocumento2 pagine2004 OrgSeminarTestAnswerGhadeer M HassanNessuna valutazione finora
- Write Your Answers On This Sheet: N O O O O O PHDocumento2 pagineWrite Your Answers On This Sheet: N O O O O O PHGhadeer M HassanNessuna valutazione finora
- Year 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestDocumento5 pagineYear 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestGhadeer M HassanNessuna valutazione finora
- ThiopheneDocumento398 pagineThiopheneAshwin MandaleNessuna valutazione finora
- Cyclisations Problems 2013Documento6 pagineCyclisations Problems 2013Ghadeer M Hassan100% (1)
- Chapter 2Documento39 pagineChapter 2Ghadeer M HassanNessuna valutazione finora
- Chapter 12 - Heterocyclic CompoundsDocumento15 pagineChapter 12 - Heterocyclic CompoundsGhadeer M HassanNessuna valutazione finora
- Indole and Benzimidiazole NucleusDocumento32 pagineIndole and Benzimidiazole NucleusSrikanth MalipatelNessuna valutazione finora
- Cycloadditions Problems 2013Documento8 pagineCycloadditions Problems 2013Ghadeer M HassanNessuna valutazione finora
- Cyclisations Solutions 2013Documento7 pagineCyclisations Solutions 2013Ghadeer M HassanNessuna valutazione finora
- Heterocycles - PART 3 - PyridazinesDocumento3 pagineHeterocycles - PART 3 - PyridazinesGhadeer M HassanNessuna valutazione finora
- Heterocycles - PART 5 - Isoxazoles and PyrazolesDocumento4 pagineHeterocycles - PART 5 - Isoxazoles and PyrazolesGhadeer M HassanNessuna valutazione finora
- Heterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsDocumento4 pagineHeterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsGhadeer M HassanNessuna valutazione finora
- Cycloadditions Solutions 2013 2Documento9 pagineCycloadditions Solutions 2013 2Ghadeer M HassanNessuna valutazione finora
- Reactivity QuinolineDocumento107 pagineReactivity QuinolineIan Otto100% (1)
- Heterocycles - PART 3 - PyridazinesDocumento3 pagineHeterocycles - PART 3 - PyridazinesGhadeer M HassanNessuna valutazione finora
- 2012 Hetero Model AnswerDocumento3 pagine2012 Hetero Model AnswerGhadeer M HassanNessuna valutazione finora
- Heterocycles - PART 5 - Isoxazoles and PyrazolesDocumento4 pagineHeterocycles - PART 5 - Isoxazoles and PyrazolesGhadeer M HassanNessuna valutazione finora
- Heterocycles - PART 6 - PyrimidinesDocumento3 pagineHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNessuna valutazione finora
- 2013 Hetero Model AnswerDocumento2 pagine2013 Hetero Model AnswerGhadeer M HassanNessuna valutazione finora
- Heterocycles - PART 6 - PyrimidinesDocumento3 pagineHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNessuna valutazione finora
- Heterocycles - PART 3 - PyridazinesDocumento3 pagineHeterocycles - PART 3 - PyridazinesGhadeer M HassanNessuna valutazione finora
- 2012 Hetero Model AnswerDocumento3 pagine2012 Hetero Model AnswerGhadeer M HassanNessuna valutazione finora
- Year 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry TestDocumento4 pagineYear 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry TestGhadeer M HassanNessuna valutazione finora
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)