Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Oxygen and Oxides
Caricato da
gkawsar220 valutazioniIl 0% ha trovato utile questo documento (0 voti)
10 visualizzazioni5 paginechemistry
Copyright
© © All Rights Reserved
Formati disponibili
DOCX, PDF o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentochemistry
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato DOCX, PDF o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
10 visualizzazioni5 pagineOxygen and Oxides
Caricato da
gkawsar22chemistry
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato DOCX, PDF o leggi online su Scribd
Sei sulla pagina 1di 5
6. Hydrogen peroxide decomposes in the presence of manganese(IV) oxide catalyst to form
water and oxygen.
2H;0,(aq) > 2H,00) + Ox)
(a) (@) What is a catalyst?
(ii) Give a test for oxygen.
q@
(0) Two experiments were performed to investigate the effect of a change in concentration
on the rate of decomposition of hydrogen peroxide at room temperature. The catalyst
had a mass of 1g and was in the form of small pellets.
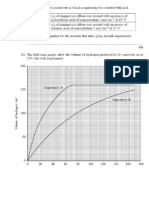
The results obtained are given in the table below.
‘Time /s 0 | 20 | 40 | 60 | 80 | 100 | 120 | 140
Experiment 1
4.cm? HO) + 46 em? H3O
Volume of oxygen produced /cm? | 0 | 20 | 34 | 44] 52 | 58 | 60 | oo
Experiment 2
6 cm? H,0) + 44 cm? HO.
Volume of oxygen produced /em? | 0 | 43 | 60 | 72 | s1 | 87 | 90 | 90
(@ Draw a labelled diagram to show how these experiments could be carried out.
Q
(ii) On the grid opposite, choose a suitable scale for the vertical axis and draw graphs
for Experiments 1 and 2.
©
(iii) Use the graph to determine the time when the reaction in Experiment 1 was half
complete.
«Ub /poonpoid waBAxo Jo sumNjo,,
140
120
100
80
40
20
Time /s
Potrebbero piacerti anche
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- Types of ExtractionDocumento5 pagineTypes of ExtractionMohammad NafaiNessuna valutazione finora
- RadiationDocumento3 pagineRadiationgkawsar22Nessuna valutazione finora
- Qualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School MuscatDocumento2 pagineQualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School Muscatgkawsar22Nessuna valutazione finora
- Report of Preparatory Mockigcse SCDocumento55 pagineReport of Preparatory Mockigcse SCgkawsar22Nessuna valutazione finora
- Classifying Materials Activity 5.1 - Physical Properties TestDocumento1 paginaClassifying Materials Activity 5.1 - Physical Properties Testgkawsar22Nessuna valutazione finora
- BondingDocumento5 pagineBondinggkawsar22Nessuna valutazione finora
- Relative Atomic MassDocumento8 pagineRelative Atomic Massgkawsar22Nessuna valutazione finora
- Acid & BaseDocumento3 pagineAcid & Basegkawsar22Nessuna valutazione finora
- Periodic TableDocumento3 paginePeriodic Tablegkawsar22Nessuna valutazione finora
- AtomsDocumento4 pagineAtomsgkawsar22Nessuna valutazione finora
- ElectrolysisDocumento7 pagineElectrolysisgkawsar22Nessuna valutazione finora
- The Aqueous Chemistry of CationsDocumento11 pagineThe Aqueous Chemistry of Cationsgkawsar22Nessuna valutazione finora
- Energy From ChemicalsDocumento4 pagineEnergy From Chemicalsgkawsar22Nessuna valutazione finora
- Crude OilDocumento4 pagineCrude Oilgkawsar22Nessuna valutazione finora
- Acid & BaseDocumento3 pagineAcid & Basegkawsar22Nessuna valutazione finora
- Test Nichrome Wire Wire Wire ColourDocumento2 pagineTest Nichrome Wire Wire Wire Colourgkawsar22Nessuna valutazione finora
- A Guide To Practical QuestionsDocumento10 pagineA Guide To Practical Questionsgkawsar22Nessuna valutazione finora
- Activity 13.3: How Does Concentration Affect Reaction Rate?Documento2 pagineActivity 13.3: How Does Concentration Affect Reaction Rate?gkawsar22Nessuna valutazione finora
- 0906 O Level Grade BoundariesDocumento2 pagine0906 O Level Grade Boundariesryuzaki589Nessuna valutazione finora
- Seperatting and AnalysisDocumento3 pagineSeperatting and Analysisgkawsar22Nessuna valutazione finora
- HeinemannIGCSE Chemistry Chapter1Documento3 pagineHeinemannIGCSE Chemistry Chapter1gkawsar22Nessuna valutazione finora
- GCSE CHEMISTRY ACIDS, BASES & SALTS HIGH DEMAND QUESTIONSDocumento21 pagineGCSE CHEMISTRY ACIDS, BASES & SALTS HIGH DEMAND QUESTIONSeeenusNessuna valutazione finora
- Rate of ReactionDocumento3 pagineRate of Reactiongkawsar22Nessuna valutazione finora
- Q 2Documento25 pagineQ 2gkawsar22Nessuna valutazione finora
- Q 1Documento36 pagineQ 1gkawsar22Nessuna valutazione finora
- Making SaltsDocumento1 paginaMaking Saltsgkawsar22Nessuna valutazione finora
- Kinetic Theory of DiffusionDocumento1 paginaKinetic Theory of Diffusiongkawsar22Nessuna valutazione finora
- Introducing Energy Changes in ReactionsDocumento2 pagineIntroducing Energy Changes in Reactionsgkawsar22Nessuna valutazione finora
- Introducing Reversible ReactionsDocumento3 pagineIntroducing Reversible Reactionsgkawsar22Nessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)