Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Exm 2012
Caricato da
api-252561013Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Exm 2012
Caricato da
api-252561013Copyright:
Formati disponibili
911640
2
SUPERVISORS USE ONLY
91164
New Zealand Qualifcations Authority, 2012. All rights reserved.
No part of this publication may be reproduced by any means without the prior permission of the New Zealand Qualifcations Authority.
ASSESSORS USE ONLY
TOTAL
Level 2 Chemistry, 2012
91164 Demonstrate understanding of bonding,
structure, properties and energy changes
9.30 am Tuesday 20 November 2012
Credits: Five
Achievement Achievement with Merit Achievement with Excellence
Demonstrate understanding of bonding,
structure, properties and energy
changes.
Demonstrate in-depth understanding
of bonding, structure, properties and
energy changes.
Demonstrate comprehensive
understanding of bonding, structure,
properties and energy changes.
Check that the National Student Number (NSN) on your admission slip is the same as the number at the
top of this page.
You should attempt ALL the questions in this booklet.
A periodic table is provided on the Resource Sheet L2CHEMR.
If you need more space for any answer, use the page(s) provided at the back of this booklet and clearly
number the question.
Check that this booklet has pages 2 11 in the correct order and that none of these pages is blank.
YOU MUST HAND THIS BOOKLET TO THE SUPERVISOR AT THE END OF THE EXAMINATION.
You are advised to spend 60 minutes answering the questions in this booklet.
QUESTION ONE
(a) Draw the Lewis structure (electron dot diagram) for each of the following molecules.
Molecule PCl
3
CO
2
H
2
S
Lewis structure
(b) The following table shows the Lewis structures and bond angles for the molecules SO
2
and H
2
CO.
Molecule SO
2
H
2
CO
Lewis structure O S O C O
H
H
Approximate bond angle
around the central atom
120 120
Explain why these molecules have different shapes, but have the same approximate bond
angle.
In your answer you should include:
the shapes of SO
2
and H
2
CO
factors which determine the shape of each molecule
an explanation of why the approximate bond angle is the same by referring to the
arrangement of electrons for each molecule.
2
Chemistry 91164, 2012
ASSESSORS
USE ONLY
3
Chemistry 91164, 2012
ASSESSORS
USE ONLY
(c) The 3-dimensional diagrams of two molecules are shown below.
C
Br
Br
Br
Br
C
Br
H
H
H
Circle the word that describes the polarity of each of the molecules CBr
4
and CH
3
Br.
CBr
4
Polar Non-polar
CH
3
Br Polar Non-polar
For each molecule, justify your choice.
4
Chemistry 91164, 2012
ASSESSORS
USE ONLY
5
This page has been deliberately left blank.
The examination continues on the following page.
Chemistry 91164, 2012
QUESTION TWO
(a) Complete the table below by stating the type of particle and the bonding (attractive forces)
between the particles for each of the substances.
Substance Type of particle Attractive forces between particles
Ammonia, NH
3
Zinc, Zn
Silicon dioxide, SiO
2
(b) Silicon dioxide has a melting point of 1770C.
Explain why silicon dioxide has a high melting point by referring to the particles and the
forces between the particles in the solid.
6
Chemistry 91164, 2012
ASSESSORS
USE ONLY
(c) Contrast both the electrical conductivity, and solubility in water, for both zinc, Zn, and
zinc chloride, ZnCl
2
, using your knowledge of structure and bonding.
7
Chemistry 91164, 2012
ASSESSORS
USE ONLY
QUESTION THREE
(a) Some Bunsen burners use methane gas, CH
4
, as a fuel. The reaction for the combustion of
methane in a Bunsen burner is shown in Equation One below.
Equation One: CH
4
+ 2O
2
CO
2
+ 2H
2
O
r
H = 889 kJ mol
1
When this reaction occurs, bonds are broken and bonds are formed.
State which bonds are broken and which bonds are formed during the reaction.
Bonds broken:
Bonds formed:
(b) Calculate the energy released when 128 g of methane is burnt.
M (CH
4
) = 16.0 g mol
1
.
(c) The equation for water boiling at 100C is shown below in Equation Two.
Equation Two: H
2
O() H
2
O(g)
r
H = 40.7 kJ mol
1
Explain why this equation is endothermic.
You should relate the energy changes that are occurring to the specifc bonds being broken or
formed.
8
Chemistry 91164, 2012
ASSESSORS
USE ONLY
(d) A student heats 72.0 g of water to 100C using a Bunsen burner.
thermometer
water
at 100C
The student then boils the water.
Calculate the mass of methane gas, CH
4
, that would need to be combusted in a Bunsen burner
to boil the 72.0 g of water.
M (H
2
O) = 18.0 g mol
1
.
In your answer you will need to:
use Equation Two to determine the amount of energy required to boil the water
use Equation One to determine the mass of methane needed to produce the required
amount of energy
assume that no energy is lost to the surrounding environment.
There is more space for your
answer to Question Three (d)
on the following page.
9
Chemistry 91164, 2012
ASSESSORS
USE ONLY
10
Chemistry 91164, 2012
ASSESSORS
USE ONLY
11
Chemistry 91164, 2012
ASSESSORS
USE ONLY
QUESTION
NUMBER
Extra paper if required.
Write the question number(s) if applicable.
9
1
1
6
4
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Chennai Sahodaya Science Set 1 QP 2022-23 - FOR PRACTICE ONLYDocumento7 pagineChennai Sahodaya Science Set 1 QP 2022-23 - FOR PRACTICE ONLYvro hamza100% (14)
- Exm 2014Documento16 pagineExm 2014api-252561013Nessuna valutazione finora
- Learning Objectives As91165Documento2 pagineLearning Objectives As91165api-252561013Nessuna valutazione finora
- Ass 2014Documento5 pagineAss 2014api-252561013Nessuna valutazione finora
- Learning Objectives As91392Documento1 paginaLearning Objectives As91392api-252561013Nessuna valutazione finora
- Exm 2014Documento12 pagineExm 2014api-252561013Nessuna valutazione finora
- As 91165Documento3 pagineAs 91165api-252561013Nessuna valutazione finora
- Ass 2013Documento6 pagineAss 2013api-252561013Nessuna valutazione finora
- Learning Objectives As91167Documento1 paginaLearning Objectives As91167api-252561013Nessuna valutazione finora
- Exm 2013Documento12 pagineExm 2013api-252561013Nessuna valutazione finora
- As 91389Documento2 pagineAs 91389api-252561013Nessuna valutazione finora
- Ass 2014Documento4 pagineAss 2014api-252561013Nessuna valutazione finora
- As 91167Documento2 pagineAs 91167api-252561013Nessuna valutazione finora
- Ass 2012Documento6 pagineAss 2012api-252561013Nessuna valutazione finora
- Learning Objectives As91161Documento1 paginaLearning Objectives As91161api-252561013Nessuna valutazione finora
- As 91161Documento2 pagineAs 91161api-252561013Nessuna valutazione finora
- Ass 2014Documento5 pagineAss 2014api-252561013Nessuna valutazione finora
- As 91390Documento3 pagineAs 91390api-252561013Nessuna valutazione finora
- Learning Objectives As91164Documento2 pagineLearning Objectives As91164api-252561013Nessuna valutazione finora
- Learning Objectives As91390Documento2 pagineLearning Objectives As91390api-252561013Nessuna valutazione finora
- Ass 2014Documento4 pagineAss 2014api-252561013Nessuna valutazione finora
- As 91393Documento2 pagineAs 91393api-252561013Nessuna valutazione finora
- As 91164Documento3 pagineAs 91164api-252561013Nessuna valutazione finora
- Learning Objectives As91391Documento4 pagineLearning Objectives As91391api-252561013Nessuna valutazione finora
- As 91435Documento3 pagineAs 91435api-271057641Nessuna valutazione finora
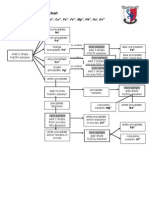
- Testing For Cations Flow ChartDocumento2 pagineTesting For Cations Flow Chartapi-252561013Nessuna valutazione finora
- Learning Objectives As91388Documento1 paginaLearning Objectives As91388api-252561013Nessuna valutazione finora
- As 91162Documento2 pagineAs 91162api-252561013Nessuna valutazione finora
- Tilting Pad BearingDocumento2 pagineTilting Pad BearingmojiryhamidNessuna valutazione finora
- Matachana Miniclave 21e - User ManualDocumento24 pagineMatachana Miniclave 21e - User Manualcarolus2009100% (1)
- Makon TD 30Documento6 pagineMakon TD 30Aji SyarifudinNessuna valutazione finora
- 21phy12 Set 1 QP Solutions29-04-2022 - 001Documento68 pagine21phy12 Set 1 QP Solutions29-04-2022 - 001Rohit KumarNessuna valutazione finora
- ds-06 Painting ProcedureDocumento7 pagineds-06 Painting Proceduremuhammad BabarNessuna valutazione finora
- Juice HeatersDocumento3 pagineJuice HeatersqqyukiNessuna valutazione finora
- Metabolic AdaptationDocumento17 pagineMetabolic AdaptationMozil Fadzil KamarudinNessuna valutazione finora
- Basics Steel ConstructionDocumento96 pagineBasics Steel ConstructionPeixuan Xu100% (1)
- Vaccum DistillationDocumento36 pagineVaccum Distillationquangquy91Nessuna valutazione finora
- Pipe FittingsDocumento63 paginePipe FittingsAdriana LimaNessuna valutazione finora
- GL3000 Chromatography System Verification Program (IQ, OQ, PQ) - ChineseToEnglishDocumento37 pagineGL3000 Chromatography System Verification Program (IQ, OQ, PQ) - ChineseToEnglish少條筋Nessuna valutazione finora
- Rubber BroDocumento19 pagineRubber BroDoloritasNessuna valutazione finora
- Parenteral S RonaDocumento14 pagineParenteral S RonalanDraynNessuna valutazione finora
- Rules of ThumbDocumento35 pagineRules of ThumbIbrahim Al-HammadiNessuna valutazione finora
- Dependence of Refractive Index On Concentration and Temperature in Electrolyte Solution, Polar Solution, Nonpolar Solution, and Protein SolutionDocumento7 pagineDependence of Refractive Index On Concentration and Temperature in Electrolyte Solution, Polar Solution, Nonpolar Solution, and Protein SolutionjoeNessuna valutazione finora
- Ana ChemDocumento1 paginaAna ChemElizer Robles Jr.Nessuna valutazione finora
- Arrhenius Equation - WikipediaDocumento25 pagineArrhenius Equation - Wikipediatirth_diwaniNessuna valutazione finora
- Hytherm S NewDocumento2 pagineHytherm S NewOliver OliverNessuna valutazione finora
- Bop (Balance of Plant)Documento4 pagineBop (Balance of Plant)hendiavianoNessuna valutazione finora
- Astm A99-03.Documento5 pagineAstm A99-03.NadhiraNessuna valutazione finora
- 2-Stoichiometry and Bacterial EnergeticsDocumento61 pagine2-Stoichiometry and Bacterial EnergeticsKiran ShresthaNessuna valutazione finora
- SHKexDocumento1 paginaSHKexC.H. LIMNessuna valutazione finora
- Biscuits FileDocumento24 pagineBiscuits Filevaishnavi rathore100% (1)
- Is 9902 2004Documento11 pagineIs 9902 2004cbbasakNessuna valutazione finora
- Water BlockingDocumento24 pagineWater BlockingramukolakiNessuna valutazione finora
- 08 Marine CableDocumento30 pagine08 Marine CableWahyu HartonoNessuna valutazione finora
- KpsDocumento3 pagineKpsJulio Rodríguez CancinoNessuna valutazione finora
- R23 LinkDocumento2 pagineR23 LinkQurban Ali RindNessuna valutazione finora
- Fastener Markings BoltsDocumento2 pagineFastener Markings BoltsmshNessuna valutazione finora