Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Levels of Protein Structure
Caricato da
chemguy31Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Levels of Protein Structure
Caricato da
chemguy31Copyright:
Formati disponibili
Levels of Protein Structure
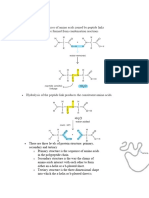
Primary structure: sequence of amino acids that forms the protein. All proteins have a
special sequence of amino acids, this sequence is derived from the cell's DNA.
(e.g. insulin )
Secondary structure: formed when the polypeptide coils or folds. This coiling and folding
forms a Alpha helix or Beta pleated sheet which are the basic form.
(e.g. actin, myosin)
Hydrogen bonds hold the coils in place. The bonds are weak, but numerous
throughout the molecule which provides stability.
Secondary structure refers to coiling and pleating of parts of the polypeptide
molecule
Tertiary structure: refers to the overall 3D structure of the final polypeptide/protein mole.
The tertiary structure forms when the secondary structure folds in on itself
(e.g. enzymes such as lipase, sucrase)
The 3D structure forms when the coils and pleats fold again. The 3D is held by
various bonds and interactions
The tertiary structure (the 3D shape is vital to the function) - e.g. enzymes and
their active site. The active site must be complementary to the substrate
molecule.
Bonds that stabilise tertiary structure are:
Disulfide bonds: Cysteine (an AA) contains sulphur. When 2 cysteines come
close together a covalent bond forms.
Ionic bonds: If the 'R' groups are charged, then oppositely charged AAs can form
ionic bonds.
Hydrogen bonds: When slightly - charged groups come into close proximity of +
charged groups, hydrogen bonds form.
Hydrophobic and Hydrophilic interactions also maintain stability in the tertiary
structure: these interactions occur in a water-based environment. Hydrophilic
AAs on the outside and hydrophobic AAs on the inside of globular proteins.
Quaternary Structure: a complex structure formed by the interaction of 2 or
more polypeptide chains. The quaternary structure is how 2 or more polypeptides form a
single protein molecule
(e g. haemoglobin)
Potrebbero piacerti anche
- ProteinsDocumento1 paginaProteinsChris_Barber09Nessuna valutazione finora
- Cell Structure, Processes, and Reproduction, Third EditionDa EverandCell Structure, Processes, and Reproduction, Third EditionNessuna valutazione finora
- Proteins: Structure and Functions ExplainedDocumento3 pagineProteins: Structure and Functions ExplainedRam Kewal TripathiNessuna valutazione finora
- Protein Structure: Primary, Secondary, Tertiary and Quaternary LevelsDocumento5 pagineProtein Structure: Primary, Secondary, Tertiary and Quaternary LevelsgangmasterNessuna valutazione finora
- shahadatDocumento5 pagineshahadatsadhinmd283Nessuna valutazione finora
- Proteins Include A Diversity of Structures, Resulting in A Wide Range of FunctionsDocumento12 pagineProteins Include A Diversity of Structures, Resulting in A Wide Range of FunctionsDanielle GuerraNessuna valutazione finora
- Bion Peptides and Proteins First Semester 2021 2022Documento55 pagineBion Peptides and Proteins First Semester 2021 2022Hashem Bani yaseenNessuna valutazione finora
- Proteins, Membranes and Cellular StructureDocumento3 pagineProteins, Membranes and Cellular StructureMarjolein van den NieuwenhuijsenNessuna valutazione finora
- Chapter 4 Cell BioDocumento22 pagineChapter 4 Cell BioJennyNessuna valutazione finora
- BiochemDocumento16 pagineBiochemRam RamNessuna valutazione finora
- Four Levels of Protein StructureDocumento2 pagineFour Levels of Protein StructureMarianne LouNessuna valutazione finora
- 1.4 ProteinDocumento55 pagine1.4 ProteinCally ChanNessuna valutazione finora
- Structure of ProteinDocumento3 pagineStructure of Proteinduaarajper4Nessuna valutazione finora
- AFN 3209 - Protein StructureDocumento8 pagineAFN 3209 - Protein StructureieunicemuthengiNessuna valutazione finora
- IndustrialDocumento18 pagineIndustrialAman KhanNessuna valutazione finora
- DNA and RNA Structure and FunctionDocumento14 pagineDNA and RNA Structure and FunctionThomas PhillipsNessuna valutazione finora
- Proteins 1-1Documento20 pagineProteins 1-1zabdullahstud1Nessuna valutazione finora
- Proteins: Structure and Functions in 40 CharactersDocumento30 pagineProteins: Structure and Functions in 40 Charactersمحمد جانNessuna valutazione finora
- 4 - Protein StructureDocumento30 pagine4 - Protein StructureInnocent TebuloNessuna valutazione finora
- Protein Structure and Function ChapterDocumento57 pagineProtein Structure and Function ChapterZeeshan ShamimNessuna valutazione finora
- Biomolecules: MacromoleculesDocumento5 pagineBiomolecules: MacromoleculesPaulaNessuna valutazione finora
- البروتيناتDocumento54 pagineالبروتيناتYaman HassanNessuna valutazione finora
- Biology RevisionDocumento2 pagineBiology RevisionamarsodhaNessuna valutazione finora
- Structure of ProteinsDocumento4 pagineStructure of ProteinsDerick SelvaNessuna valutazione finora
- Lecture 3-ProteinsDocumento9 pagineLecture 3-ProteinsOminousCroakNessuna valutazione finora
- Molecular Protien StuctureDocumento5 pagineMolecular Protien Stucturechetan0047Nessuna valutazione finora
- Protein Structure: Peptide Bond FormationDocumento9 pagineProtein Structure: Peptide Bond FormationAndreaGilRuizNessuna valutazione finora
- Secondary (2º) StructureDocumento13 pagineSecondary (2º) Structurebenina biancaNessuna valutazione finora
- Peptide Bond Formation of Polypeptides and ProteinDocumento11 paginePeptide Bond Formation of Polypeptides and ProteinSusana N peNessuna valutazione finora
- Enzymes 1Documento1 paginaEnzymes 1Food & HealthNessuna valutazione finora
- ProteinsDocumento33 pagineProteinsLena WęglarzNessuna valutazione finora
- Module 10: ProteinsDocumento2 pagineModule 10: ProteinsMariam EskandariNessuna valutazione finora
- All biomolecules are macro or large polymersDocumento49 pagineAll biomolecules are macro or large polymersAndrea KuardatNessuna valutazione finora
- Lecture 2 - BiomoleculesDocumento55 pagineLecture 2 - BiomoleculesRamkiNessuna valutazione finora
- Tertiary Structure of ProteinDocumento11 pagineTertiary Structure of ProteinCindy LeeNessuna valutazione finora
- Proteins: Proteins Are Large Biomolecules, or Macromolecules, Consisting of One orDocumento15 pagineProteins: Proteins Are Large Biomolecules, or Macromolecules, Consisting of One orKaleem KhanNessuna valutazione finora
- Proteins - Many Structures, Many FunctionsDocumento30 pagineProteins - Many Structures, Many FunctionsXEDGER09Nessuna valutazione finora
- Understanding Proteins from Primary to Quaternary StructureDocumento10 pagineUnderstanding Proteins from Primary to Quaternary StructureDarshan RNessuna valutazione finora
- Proteins: NH (Amine) Group COOH (Carboxylic) GroupDocumento5 pagineProteins: NH (Amine) Group COOH (Carboxylic) GroupPriyanthi FernandoNessuna valutazione finora
- Chemistry of Amino Acid and Nucleic Acid - 060921Documento61 pagineChemistry of Amino Acid and Nucleic Acid - 060921Samuella Cecilia Rikadona PurbaNessuna valutazione finora
- Department of Industrial Chemistry: Bahir Dar UniversityDocumento25 pagineDepartment of Industrial Chemistry: Bahir Dar UniversityFikere'ab HabtamuNessuna valutazione finora
- ProteinsDocumento9 pagineProteinsJada HartNessuna valutazione finora
- Week 5Documento21 pagineWeek 5ML MLNessuna valutazione finora
- Lecture - ProteinsDocumento62 pagineLecture - ProteinsBahesty Monfared AkashNessuna valutazione finora
- Note For EJU 30Documento4 pagineNote For EJU 30mr.draungnaingwinNessuna valutazione finora
- DNA and RNA Structure, Functions of Biomolecules, Protein StructureDocumento4 pagineDNA and RNA Structure, Functions of Biomolecules, Protein StructureSaksham WadhwaNessuna valutazione finora
- 3.3C: Protein Structure: Learning ObjectivesDocumento5 pagine3.3C: Protein Structure: Learning ObjectivesBriannaCarpenterNessuna valutazione finora
- Quaternary StructureDocumento2 pagineQuaternary StructureJane ConstantinoNessuna valutazione finora
- Module 5. Proteins Course Outcomes: at The End of The Course, The Student Shall Be Able ToDocumento6 pagineModule 5. Proteins Course Outcomes: at The End of The Course, The Student Shall Be Able ToAldine MabulacNessuna valutazione finora
- Extracellular MatrixDocumento4 pagineExtracellular MatrixElena OlmedoNessuna valutazione finora
- 3D Structures of ProteinDocumento11 pagine3D Structures of ProteinSheila GarciaNessuna valutazione finora
- Jeremiah Whyte: Biology AssignmentDocumento5 pagineJeremiah Whyte: Biology AssignmentJeremiah WhyteNessuna valutazione finora
- BIOLOGY FOR ENGINEERS Semester I BiotechnologyDocumento22 pagineBIOLOGY FOR ENGINEERS Semester I BiotechnologySneeha VeerakumarNessuna valutazione finora
- 08 - Peptides and ProteinsDocumento5 pagine08 - Peptides and ProteinsMiguel BañosNessuna valutazione finora
- Level of Organisation of Protein StructureDocumento18 pagineLevel of Organisation of Protein Structureyinghui94Nessuna valutazione finora
- Building Blocks of Life Student Edition CIBT Zl8a60Documento15 pagineBuilding Blocks of Life Student Edition CIBT Zl8a60Jcob SntosNessuna valutazione finora
- Biology Revision NotesDocumento42 pagineBiology Revision Noteshumza bhattiNessuna valutazione finora
- Biological Molecules Spec Notes KLMDocumento2 pagineBiological Molecules Spec Notes KLMAdam EchikrNessuna valutazione finora
- Protein Biochemistry: A Brief IntroductionDocumento22 pagineProtein Biochemistry: A Brief IntroductionbiochemiNessuna valutazione finora