Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Jc130607 Additional Helpful Info For The PDF
Caricato da
joebug34Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Jc130607 Additional Helpful Info For The PDF
Caricato da
joebug34Copyright:
Formati disponibili
ADDITIONAL HELPFUL INFO FOR THE DRUG MODIFICATION EXPERIMENT CALCULATIONS (CHEM& 131, SPSCC, J.
Chen, B234)
The parent drug is CPZ-HCl (FW = 355.3 g/mol). For the final mCPZ product, remove the HCl from the parent drug, replace the H with CH3 and replace the Cl with I (remember, we used iodomethane, and all of the atoms in iodomethane are being incorporated to make the final mCPZ product). Since it is difficult to find info for mCPZ online, you probably will need to calculate the formula weight of mCPZ by hand, using the atomic mass values from the periodic table for the elements in the compound. To calculate the number of moles of carbon per compound, multiply the moles of compound by the number of carbons in that compound. For example, 1.00 mole of ethanol contains 2.00 moles of carbon, since there are 2 carbons per ethanol. Add up all of these moles of carbon to get the total number of moles of carbon used. To calculate the number of liters of carbon dioxide generated by the carbon used in the experiment: Assume that every one mole of carbon generated as waste is converted to one mole of carbon dioxide once the waste has been incinerated. THEN, assume that this carbon dioxide is an ideal gas that occupies 22.4 liters of volume for every mole of CO2.
Heres a thought question you should consider trying to answer in Module 3: Why do you think the mCPZ spot has such a high Rf value, while the CPZ spot has such a low Rf value, given that their chemical structures really are not THAT different? Heres a hint: There was ammonia in the TLC solvent, which RAISES the pH of the solvent. What does a high pH do to the CPZ-HCl?
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Structural Engineering Formulas Second EditionDocumento224 pagineStructural Engineering Formulas Second Editionahmed_60709595194% (33)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Tom Rockmore - Hegel's Circular EpistemologyDocumento213 pagineTom Rockmore - Hegel's Circular Epistemologyluiz100% (1)
- SAMPLE Forklift Safety ProgramDocumento5 pagineSAMPLE Forklift Safety ProgramSudiatmoko SupangkatNessuna valutazione finora
- Citadel Securities Australia Pty LTD - Company DetailsDocumento5 pagineCitadel Securities Australia Pty LTD - Company DetailsBrendan OswaldNessuna valutazione finora
- Self-Actualization in Robert Luketic'S: Legally Blonde: A HumanisticDocumento10 pagineSelf-Actualization in Robert Luketic'S: Legally Blonde: A HumanisticAyeshia FréyNessuna valutazione finora
- Lesson Plan Ordinal NumbersDocumento5 pagineLesson Plan Ordinal Numbersapi-329663096Nessuna valutazione finora
- Islamophobia: The Roots of IntoleranceDocumento7 pagineIslamophobia: The Roots of Intolerancejoebug34Nessuna valutazione finora
- Joe Was A Toddler He Had A Formula For Everlasting Life Joe Sucked He Died Alone Ye (Documento1 paginaJoe Was A Toddler He Had A Formula For Everlasting Life Joe Sucked He Died Alone Ye (joebug34Nessuna valutazione finora
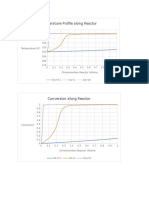
- Temperature Profile Along ReactorDocumento3 pagineTemperature Profile Along Reactorjoebug34Nessuna valutazione finora
- Bib 5390Documento1 paginaBib 5390joebug34Nessuna valutazione finora
- ChE 334 Appendix CodeDocumento4 pagineChE 334 Appendix Codejoebug34Nessuna valutazione finora
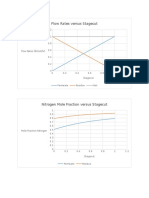
- Pressure Ratio and Conversion Versus Catalyst WeightDocumento5 paginePressure Ratio and Conversion Versus Catalyst Weightjoebug34Nessuna valutazione finora
- Math423 HW#Documento1 paginaMath423 HW#joebug34Nessuna valutazione finora
- Table of Van Der Waals ConstantsDocumento2 pagineTable of Van Der Waals Constantsjoebug34Nessuna valutazione finora
- Poop A Doop DoopsDocumento1 paginaPoop A Doop Doopsjoebug34Nessuna valutazione finora
- H2o2 ApDocumento12 pagineH2o2 Apjoebug34Nessuna valutazione finora
- California Academy For Lilminius (Cal) : Lesson PlanDocumento4 pagineCalifornia Academy For Lilminius (Cal) : Lesson Plandarryl franciscoNessuna valutazione finora
- Pamphlet On Arrangement of Springs in Various Casnub Trolleys Fitted On Air Brake Wagon PDFDocumento9 paginePamphlet On Arrangement of Springs in Various Casnub Trolleys Fitted On Air Brake Wagon PDFNiKhil GuPtaNessuna valutazione finora
- (Official) AVTC5 - Unit 1 - Before ClassDocumento11 pagine(Official) AVTC5 - Unit 1 - Before ClassNhân NguyễnNessuna valutazione finora
- The English We SpeakDocumento2 pagineThe English We Speakcaeronmustai100% (1)
- Ashfaque Ahmed-The SAP Materials Management Handbook-Auerbach Publications, CRC Press (2014)Documento36 pagineAshfaque Ahmed-The SAP Materials Management Handbook-Auerbach Publications, CRC Press (2014)surajnayak77Nessuna valutazione finora
- D4462045416 PDFDocumento3 pagineD4462045416 PDFSamir MazafranNessuna valutazione finora
- Month Puzzle Two VariableDocumento6 pagineMonth Puzzle Two VariableNayan KaithwasNessuna valutazione finora
- Atlantis Implant Compatibility Chart 79214-US-1107Documento2 pagineAtlantis Implant Compatibility Chart 79214-US-1107Jean-Christophe PopeNessuna valutazione finora
- Electrostatics Practice ProblemsDocumento4 pagineElectrostatics Practice ProblemsMohammed Aftab AhmedNessuna valutazione finora
- 12.3 What Is The Nomenclature System For CFCS/HCFCS/HFCS? (Chemistry)Documento3 pagine12.3 What Is The Nomenclature System For CFCS/HCFCS/HFCS? (Chemistry)Riska IndriyaniNessuna valutazione finora
- Climate Declaration: For White Corex PlasterboardDocumento1 paginaClimate Declaration: For White Corex PlasterboardAbdullah BeckerNessuna valutazione finora
- Roles of Community Health NursingDocumento2 pagineRoles of Community Health Nursingdy kimNessuna valutazione finora
- Unit 12 BriefDocumento7 pagineUnit 12 Briefapi-477397447Nessuna valutazione finora
- Fluid Mechanics HydraulicsDocumento420 pagineFluid Mechanics Hydraulicsanonymousdi3noNessuna valutazione finora
- British Airways Culture and StructureDocumento29 pagineBritish Airways Culture and Structure陆奕敏Nessuna valutazione finora
- Application Tracking System: Mentor - Yamini Ma'AmDocumento10 pagineApplication Tracking System: Mentor - Yamini Ma'AmBHuwanNessuna valutazione finora
- Bach Polonaise G Min BWV 119 A4Documento1 paginaBach Polonaise G Min BWV 119 A4vincenzovaiaNessuna valutazione finora
- Task 3: New - HTMLDocumento12 pagineTask 3: New - HTMLGONELA SAI LOKESH (RA2011028010100)Nessuna valutazione finora
- Chemistry Previos Papaer 313Documento19 pagineChemistry Previos Papaer 313Ashu GuptaNessuna valutazione finora
- Learning Activity No.2Documento1 paginaLearning Activity No.2Miki AntonNessuna valutazione finora
- Complete DaikinDocumento11 pagineComplete DaikinAGNIDEEP BAIDYANessuna valutazione finora
- Different Art TechniquesDocumento39 pagineDifferent Art TechniquesRommel LegaspiNessuna valutazione finora
- Ip TunnelingDocumento15 pagineIp TunnelingBon Tran HongNessuna valutazione finora
- Isp List MatiurDocumento3 pagineIsp List Matiurmatiur7Nessuna valutazione finora