Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Wiki Media Periodic
Caricato da
Dann DomeCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Wiki Media Periodic
Caricato da
Dann DomeCopyright:
Formati disponibili
PERIODIC TABLE OF THE ELEMENTS
GROUP
IA
1
1
1.0079
1
1s
HYDROGEN
6.941(2)
1
[He] 2s
Li
LITHIUM
11
22.990
1
[Ne] 3s
19
39.098
1
[Ar] 4s
POTASSIUM
37
85.468
1
[Kr] 5s
Rb
RUBIDIUM
55
132.905
1
[Xe] 6s
FAMILY
2
4
(223)
1
[Rn] 7s
ATOMIC NUMBER
[He] 2s
78
ELECTRON
CONFIGURATION (3)
12
24.305
2
[Ne] 3s
MAGNESIUM
20
40.078
2
[Ar] 4s
Ca
CALCIUM
38
87.62(1)
2
[Kr] 5s
Sr
STRONTIUM
56
137.327
2
[Xe] 6s
Ba
BARIUM
88
(226)
2
[Rn] 7s
Fr
Ra
FRANCIUM
RADIUM
3
21
IIIB
44.956
1
[Ar] 3d 4s
Sc
SCANDIUM
39
88.906
1
[Kr] 4d 5s
YTTRIUM
57-71
Lanthanides
89-103
Actinides
IVB
4
22
47.867
2
[Ar] 3d 4s
[Kr] 4d 5s
Zr
178.49(2)
14
VB
5
23
50.942
3
41
Hf
HAFNIUM
104
(261)
Rf
RUTHERFORDIUM
138.905
1
[Xe] 5d 6s
La
LANTHANUM
Halogens
Transition metals
Noble gases
Physical State (25C. 1 atm)
Actinides
Ne
Hg - liquid
42
95.94(2)
5
43
8
26
55.845
6
[Ar] 3d 4s
(98)
6
VIIIB
74
183.84(1)
14
75
14
76
10
195.084
14
13
48
80
Ta
105
(262)
DUBNIUM
(227)
1
[Rn] 6d 7s
10
Ce
CERIUM
Ac
ACTINIUM
(264)
Bh
108
(277)
HASSIUM
(268)
110
(281)
Au
111
MERCURY
(272)
112
49
PHOSPHORUS
33
74.922
10
16
17
35
18
39.948
2
Ar
ARGON
79.904
10
20.180
[Ne] 3s 3p
CHLORINE
78.96(3)
10
NEON
Cl
SULFUR
HELIUM
Ne
35.453
2
He
[He] 2s 2p
[Ne] 3s 3p
S
10
32.065
34
FLUORINE
[Ne] 3s 3p
72.64(1)
10
18.998
[He] 2s 2p
30.974
2
OXYGEN
[Ne] 3s 3p
GERMANIUM
114.818
10
In
Hg
GOLD
32
15
[He] 2s 2p
VIIA
36
83.798
10
50
118.710
10
Se
As
ARSENIC
51
SELENIUM
121.760
10
Br
BROMINE
52 127.60(3) 53
10
Kr
KRYPTON
126.904
10
54
131.293
10
Sn
(285)
81
204.383
14
10
[Xe] 4f 5d 6s 6p
Tl
THALLIUM
113
Sb
(284)
82
207.2(1)
14
10
[Xe] 4f 5d 6s 6p
Pb
LEAD
114
Te
ANTIMONY
TIN
83
TELLURIUM
208.980
14
10
[Xe] 4f 5d 6s 6p
Bi
BISMUTH
(289)
115
(288)
84
(209)
14
10
[Xe] 4f 5d 6s 6p
Po
POLONIUM
116
(292)
Xe
IODINE
85
XENON
(210)
14
10
[Xe] 4f 5d 6s 6p
At
ASTATINE
117
86
(222)
14
10
[Xe] 4f 5d 6s 6p
Rn
RADON
118
(294)
Ds Rg Cn Uut Uuq Uup Uuh Uus* Uuo
MEITNERIUM DARMSTADTIUM ROENTGENIUM COPERNICIUM UNUNTRIUM UNUNQUADIUM UNUNPENTIUM UNUNHEXIUM UNUNSEPTIUM UNUNOCTIUM
59
140.908
3
[Xe] 4f 6s
60
144.242
4
[Xe] 4f 6s
61
(145)
5
[Xe] 4f 6s
[Xe] 4f 6s
231.036
2
92
238.029
3
93
(237)
4
[Rn] 5f 6d 7s [Rn] 5f 6d 7s [Rn] 5f 6d 7s
THORIUM
PROTACTINIUM
Pa
151.964
7
[Xe] 4f 6s
64 157.25(3) 65
7
URANIUM
SAMARIUM
94
(244)
6
[Rn] 5f 7s
EUROPIUM
95
(243)
7
158.925
9
[Xe] 4f 6s
GADOLINIUM
TERBIUM
96
(247)
7
[Rn] 5f 7s
[Rn] 5f 6d 7s
AMERICIUM
CURIUM
Tb
97
(247)
9
[Rn] 5f 7s
Np Pu Am Cm Bk
NEPTUNIUM PLUTONIUM
[Xe] 4f 5d 6s
Nd Pm Sm Eu Gd
Pr
91
62 150.36(2) 63
6
[Rn] 6d 7s
Th
109
PLATINUM
Mt
Hs
BOHRIUM
Pt
IRIDIUM
PRASEODYMIUM NEODYMIUM PROMETHIUM
232.038
2
107
Ir
OSMIUM
15.999
17
9
(1) Pure & Applied Chemistry, Vol. 78,
No. 11, pp. 20512066 (2006)
http://www.iupac.org/publications/pac/2006/pdf/7811x2051.pdf
[Xe] 4f 5d 6s
90
(266)
SEABORGIUM
140.116
1
106
Os
RHENIUM
Sg
Db
58
Re
TUNGSTEN
TANTALUM
INDIUM
200.59(2)
14
VIA
16
8
[Kr] 4d 5s 5p [Kr] 4d 5s 5p [Kr] 4d 5s 5p [Kr] 4d 5s 5p [Kr] 4d 5s 5p [Kr] 4d 5s 5p
Cd
10
28.086
SILICON
Si
69.723
NITROGEN
[Ne] 3s 3p
GALLIUM
112.411
10
14
14.007
[He] 2s 2p
Ga Ge
CADMIUM
196.967
ALUMINUM
10
AI
31
VA
15
7
[Ar] 3d 4s 4p [Ar] 3d 4s 4p [Ar] 3d 4s 4p [Ar] 3d 4s 4p [Ar] 3d 4s 4p [Ar] 3d 4s 4p
[Kr] 4d 5s
Ag
14
10
ZINC
SILVER
79
65.409
Zn
107.868
10
IIB
12
30
12.011
CARBON
26.982
2
IVA
14
6
[He] 2s 2p
[Ne] 3s 3p
[Ar] 3d 4s
[Kr] 4d 5s
Pd
78
COPPER
46 106.42(1) 47
PALLADIUM
2
63.546
Cu
[Kr] 4d
192.217
IB
11
29
[Ar] 3d 4s
10
Rh
14
NICKEL
RHODIUM
77
58.693
Ni
102.906
8
10
28
Fe
Tc - Man-made
[Ar] 3d 4s
[Kr] 4d 5s
190.23(3)
14
COBALT
IRON
44 101.07(2) 45
Ru
186.207
Co
[Kr] 4d 5s
Tc
58.933
[Ar] 3d 4s
Fe
7
[Kr] 4d 5s
9
27
BORON
- solid
- gas
10.811
[He] 2s 2p
Lanthanides
MOLYBDENUM TECHNETIUM RUTHENIUM
180.947
14
54.938
Mn
Nb Mo
NIOBIUM
73
VIIB
[Ar] 3d 4s
[Kr] 4d 5s
ACTINIDES
d
Alkaline earth metals
IIIA
13
5
[Xe] 4f 5d 6s [Xe] 4f 5d 6s [Xe] 4f 5d 6s [Xe] 4f 5d 6s [Xe] 4f 5d 6s [Xe] 4f 5d 6s [Xe] 4f 5d 6s [Xe] 4f 5d 6s [Xe] 4f 5d 6s
57
7
25
CHROMIUM MANGANESE
LANTHANIDES
51.996
Cr
92.906
4
VIB
6
24
[Ar] 3d 4s
[Kr] 4d 5s
ZIRCONIUM
72
ELEMENT NAME
Non-metal
Chalcogens
PLATINUM
VANADIUM
91.224
Metalloids
Alkaline metals
Pt
TITANIUM
2
[Ar] 3d 4s
Ti
40
195.084
14
1s
Metal
(1)(2)
[Xe] 4f 5d 6s
ATOMIC
SYMBOL
89
ORBITAL
FILLING
RELATIVE
ATOMIC MASS
(g.mol-1)
BERYLLIUM
Cs
87
ELECTRONEGATIVITY
9.0122
Be
CESIUM
IIA
Na Mg
SODIUM
18 VIIIA
2 4.0026
66
162.500
10
[Xe] 4f 6s
Dy
DYSPROSIUM
98
(251)
10
[Rn] 5f 7s
Cf
67
164.930
11
[Xe] 4f 6s
Ho
HOLMIUM
99
(252)
11
[Rn] 5f 7s
68
167.259
12
[Xe] 4f 6s
Er
13
THULIUM
(257)
12
168.934
[Xe] 4f 6s
70 173.04(3) 71
14
[Xe] 4f 6s
[Rn] 5f 7s
101
(258)
13
[Rn] 5f 7s
YTTERBIUM
102
(259)
14
FERMIUM
[Rn] 5f 7s
Es Fm Md No
BERKELIUM CALIFORNIUM EINSTEINIUM
Tm Yb
ERBIUM
100
69
MENDELEVIUM NOBELIUM
14
Lu
LUTETIUM
103
(262)
Lr
LAWRENCIUM
(2) The relative atomic mass is given with five significant digits. For items that do not have a stable radionuclide, the value in parentheses indicates the mass number of the isotope of the element with the longest half-life.
However, the three elements Th, Pa and Pu which have a characteristic terrestrial isotopic composition, an atomic weight is indicated.
(3) The electronic configurations for which there is doubt are not given.
174.967
[Xe] 4f 5d 6s
Potrebbero piacerti anche
- Materials Data for Cyclic Loading: Low-Alloy SteelsDa EverandMaterials Data for Cyclic Loading: Low-Alloy SteelsValutazione: 5 su 5 stelle5/5 (2)
- ThermodynamicsDocumento2 pagineThermodynamicsDann DomeNessuna valutazione finora
- Unusual Structures and Physical Properties in Organometallic ChemistryDa EverandUnusual Structures and Physical Properties in Organometallic ChemistryNessuna valutazione finora
- Wiki Media PeriodicDocumento1 paginaWiki Media PeriodicSepehr Masoumi-AlamoutiNessuna valutazione finora
- Atomic Properties of The Elements TableDocumento1 paginaAtomic Properties of The Elements TableMaahiNessuna valutazione finora
- Metallabenzenes: An Expert ViewDa EverandMetallabenzenes: An Expert ViewL. James WrightNessuna valutazione finora
- Periodic TableDocumento13 paginePeriodic TablenithyachatsuNessuna valutazione finora
- Application of IC-MS and IC-ICP-MS in Environmental ResearchDa EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiNessuna valutazione finora
- Periodic Table: ChemistryDocumento1 paginaPeriodic Table: ChemistryRafael RamosNessuna valutazione finora
- Heterogeneous Catalysis at Nanoscale for Energy ApplicationsDa EverandHeterogeneous Catalysis at Nanoscale for Energy ApplicationsNessuna valutazione finora
- IA IIA IiibivbvbvibviibviiibibiibDocumento7 pagineIA IIA IiibivbvbvibviibviiibibiibAmalia maysarah asharNessuna valutazione finora
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyDa EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNessuna valutazione finora
- Periodic Table of The Elements: M. K. MistryDocumento2 paginePeriodic Table of The Elements: M. K. MistryxanshahNessuna valutazione finora
- Experimental and Theoretical Approaches to Actinide ChemistryDa EverandExperimental and Theoretical Approaches to Actinide ChemistryJohn K. GibsonNessuna valutazione finora
- Complete Periodic-TableDocumento16 pagineComplete Periodic-Tableapi-234891239Nessuna valutazione finora
- Group: 1 IA 18 ViiiaDocumento1 paginaGroup: 1 IA 18 ViiiaJosé Larragaña OsunaNessuna valutazione finora
- 1.1.0.1.2 SR Periodic-Table f11Documento1 pagina1.1.0.1.2 SR Periodic-Table f11Aboahmed AliNessuna valutazione finora
- Tabla PeriodicaDocumento1 paginaTabla PeriodicaKatherine VelasquezNessuna valutazione finora
- Elements Arranged in Terms of Atomic NumberDocumento10 pagineElements Arranged in Terms of Atomic NumbergopuvenkatNessuna valutazione finora
- Chemistry PropertiesDocumento7 pagineChemistry PropertiessphereofmatterNessuna valutazione finora
- Electron Configuration Chart - NH's PageDocumento5 pagineElectron Configuration Chart - NH's PageMalik Hamza AslamNessuna valutazione finora
- Logam Alkali Alkali Tanah Lantanida Aktinida Logam Transisi Logam Metaloid Nonlogam Halogen Gas MuliaDocumento10 pagineLogam Alkali Alkali Tanah Lantanida Aktinida Logam Transisi Logam Metaloid Nonlogam Halogen Gas MuliaANGGINessuna valutazione finora
- Tabel Periodic IIDocumento1 paginaTabel Periodic IIMada Drnn100% (1)
- Tabel KimiaDocumento9 pagineTabel Kimiaendia verniNessuna valutazione finora
- Logam Alkali Alkali Tanah Lantanida Aktinida Logam Transisi: Deret Kimia Tabel PeriodikDocumento9 pagineLogam Alkali Alkali Tanah Lantanida Aktinida Logam Transisi: Deret Kimia Tabel PeriodikRatasi MessiNessuna valutazione finora
- Deret Kimia Tabel PeriodikDocumento8 pagineDeret Kimia Tabel Periodikas100% (1)
- Hsslive-Xii-Chem-8. The D & F Block ElementsDocumento16 pagineHsslive-Xii-Chem-8. The D & F Block ElementsHakim AbbasNessuna valutazione finora
- Electron Configurations of The ElementsDocumento2 pagineElectron Configurations of The ElementsLaura lau100% (1)
- UntitledDocumento1 paginaUntitledKaoru OtsukaNessuna valutazione finora
- List of Elements by Atomic Properties - WikipediaDocumento7 pagineList of Elements by Atomic Properties - WikipediaShahid AhmedNessuna valutazione finora
- Elements CHEMISTRY 5Documento27 pagineElements CHEMISTRY 5Nick FullerNessuna valutazione finora
- Deret Kimia Tabel PeriodikDocumento10 pagineDeret Kimia Tabel PeriodikAdhi D'child StgNessuna valutazione finora
- Element Atomic Number Element Symbol Element Name Element Electron ConfigurationDocumento5 pagineElement Atomic Number Element Symbol Element Name Element Electron ConfigurationShashwat SinghNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic TableJoeniar RasmawanNessuna valutazione finora
- Jadual Berkala KimiaDocumento2 pagineJadual Berkala KimialuklukzubirNessuna valutazione finora
- Periodic Table of The Elements: Be B C LiDocumento1 paginaPeriodic Table of The Elements: Be B C LiTamara KhasimaNessuna valutazione finora
- Periodic Table ColorDocumento1 paginaPeriodic Table ColorRoberto TanakaNessuna valutazione finora
- Bishop Periodic Table PDFDocumento1 paginaBishop Periodic Table PDFzelNessuna valutazione finora
- Bishop Periodic TableDocumento1 paginaBishop Periodic TableAashay PatilNessuna valutazione finora
- Periodic Table With Several InfosDocumento1 paginaPeriodic Table With Several InfosBCLNessuna valutazione finora
- Nama Lambang Nomor Atom Massa Atom: Logam Alkali Alkali Tanah Lantanida Aktinida Logam TransisiDocumento9 pagineNama Lambang Nomor Atom Massa Atom: Logam Alkali Alkali Tanah Lantanida Aktinida Logam TransisisherleyNessuna valutazione finora
- Chem IsDocumento22 pagineChem IsKeyman Rahmat TNessuna valutazione finora
- The Periodic Table NotesDocumento23 pagineThe Periodic Table Notesapi-239426184Nessuna valutazione finora
- Electron AffinityDocumento10 pagineElectron AffinityCesarPazoNessuna valutazione finora
- Webelements Table 5sf 2012-06-07Documento0 pagineWebelements Table 5sf 2012-06-07api-239300177Nessuna valutazione finora
- Periodictable BWDocumento1 paginaPeriodictable BWShubham SinghNessuna valutazione finora
- Periodic Table of The Elements: C Be B Al S LiDocumento2 paginePeriodic Table of The Elements: C Be B Al S LiAndres FacuNessuna valutazione finora
- BDH Periodic Table of The Elements PosterDocumento1 paginaBDH Periodic Table of The Elements PosterCuauhtemoc MoctezumaNessuna valutazione finora
- Periodic Table DizzyDocumento1 paginaPeriodic Table DizzyazizahdwiNessuna valutazione finora
- 바닥상태 전자배치와 원자가전자Documento3 pagine바닥상태 전자배치와 원자가전자a01042932313Nessuna valutazione finora
- Periodni Sistem Elemenata PDFDocumento1 paginaPeriodni Sistem Elemenata PDFmralienNessuna valutazione finora
- Configuraciones Electrónicas AbreviadasDocumento12 pagineConfiguraciones Electrónicas AbreviadasEVELYN CCASA ECHEVARRIANessuna valutazione finora
- Atomic Weights of The Elements 2009Documento8 pagineAtomic Weights of The Elements 2009Balaram mondalNessuna valutazione finora
- 2011 Atomic WeightsDocumento8 pagine2011 Atomic WeightsakvssakthivelNessuna valutazione finora
- PeriodicTableOfTheElementsBW PDFDocumento1 paginaPeriodicTableOfTheElementsBW PDFAsyraf ZawawiNessuna valutazione finora
- Periodictable PsDocumento1 paginaPeriodictable PskgrhoadsNessuna valutazione finora
- Unsur Padat KimiaDocumento8 pagineUnsur Padat Kimiaadi setyaNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic TableLiyana ShafiqahNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic Tableangel_personalNessuna valutazione finora
- PhysicsDocumento4 paginePhysicsNelmart SolteoNessuna valutazione finora
- Tensile TestDocumento11 pagineTensile TestOsura GunasenaNessuna valutazione finora
- 8-Heat Transfer-2016 - Ans Key-Master FileDocumento12 pagine8-Heat Transfer-2016 - Ans Key-Master FileLourdes Cagungun100% (2)
- Water Softener ResinDocumento3 pagineWater Softener Resinmkgchem100% (1)
- Acgih Manual 1998 (401-500)Documento100 pagineAcgih Manual 1998 (401-500)HéctorNessuna valutazione finora
- ChemDocumento2 pagineChemDBANJAN MAITYNessuna valutazione finora
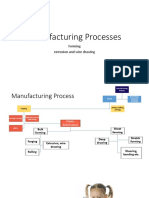
- Slide 11 Extrusion and Wire DrawingDocumento53 pagineSlide 11 Extrusion and Wire Drawingjohn doeNessuna valutazione finora
- Method Statement For Painting Works: MS Number Revision Date-IssuedDocumento9 pagineMethod Statement For Painting Works: MS Number Revision Date-IssuedKalai ManiNessuna valutazione finora
- Unit-I Chemical Bonding and Molecular Structure: (18 Contact Hours)Documento3 pagineUnit-I Chemical Bonding and Molecular Structure: (18 Contact Hours)Imran Afzal BhatNessuna valutazione finora
- Titrimetric Methods of AnalysesDocumento10 pagineTitrimetric Methods of AnalysesJason BakerNessuna valutazione finora
- Sampling Plan PDFDocumento3 pagineSampling Plan PDFamitNessuna valutazione finora
- Makrolon Solid Flame Retardant Polycarbonate Sheet: Your BenefitsDocumento2 pagineMakrolon Solid Flame Retardant Polycarbonate Sheet: Your BenefitsAbdelmajid HmNessuna valutazione finora
- Determination of In-Situ Unit Weight of Soil: Experiment No 2 & 3 Soil Mechanics Laboratory CE PC 594Documento30 pagineDetermination of In-Situ Unit Weight of Soil: Experiment No 2 & 3 Soil Mechanics Laboratory CE PC 594SumanHaldar100% (1)
- Keenagoda BOQ FinalDocumento30 pagineKeenagoda BOQ FinalChamin Subhawickrama50% (2)
- SM-204-TechReport 01 2022 AnsichtDocumento4 pagineSM-204-TechReport 01 2022 AnsichtDanny DoanNessuna valutazione finora
- Rmi Verif RC Columns 3 2Documento76 pagineRmi Verif RC Columns 3 2Fernando MartinezNessuna valutazione finora
- 7.bending Moments in BeamsDocumento7 pagine7.bending Moments in BeamsMoiz AmirNessuna valutazione finora
- Elvacite® 2016 Acrylic Resin: ApplicationsDocumento4 pagineElvacite® 2016 Acrylic Resin: ApplicationsPaola Lopez100% (2)
- CH202 Midterm 1 2024 - Info and PracticeDocumento10 pagineCH202 Midterm 1 2024 - Info and Practiceaurorascoh20Nessuna valutazione finora
- Structural Design of Flexible Pavement-ATJ 5-85 Pindaan 2013Documento38 pagineStructural Design of Flexible Pavement-ATJ 5-85 Pindaan 2013Mohammad Yunus Salehi67% (3)
- Guide EU RoHS Exemption List PC GD 200625Documento32 pagineGuide EU RoHS Exemption List PC GD 200625canacNessuna valutazione finora
- Cambridge Secondary 1 Checkpoint: Cambridge Assessment International EducationDocumento16 pagineCambridge Secondary 1 Checkpoint: Cambridge Assessment International EducationAnisahNessuna valutazione finora
- SimXpert R3.2 Crash Workspace GuideDocumento344 pagineSimXpert R3.2 Crash Workspace Guidepaulkastle100% (1)
- Hxe ImDocumento61 pagineHxe ImHồ Viết DuyNessuna valutazione finora
- Concrete NDT SeminarDocumento62 pagineConcrete NDT SeminarlokeshlokNessuna valutazione finora
- OEM Mechanical SealsDocumento63 pagineOEM Mechanical Sealsparatrpr2003100% (1)
- MTPDF4 - Module 4 Main PDF LessonDocumento35 pagineMTPDF4 - Module 4 Main PDF LessonEunnicePanaliganNessuna valutazione finora
- Controlled Polymers For Pigment DispersantsDocumento9 pagineControlled Polymers For Pigment Dispersantstrường phạmNessuna valutazione finora
- Megha Engineering & Infrastructures LTD: List of Register TechnicalDocumento31 pagineMegha Engineering & Infrastructures LTD: List of Register TechnicalLaxmikanta swainNessuna valutazione finora
- Science Paper 3 - SolvedDocumento10 pagineScience Paper 3 - SolvedRavi KumarNessuna valutazione finora